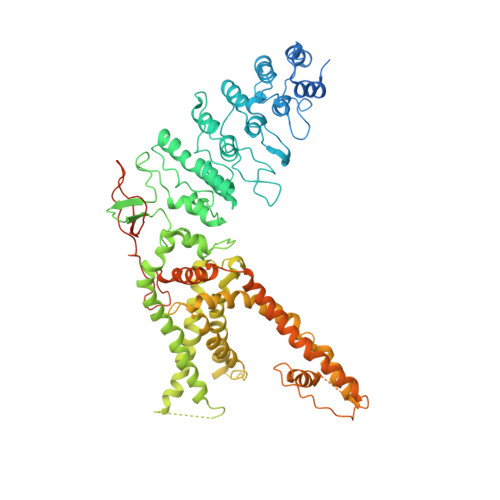
Structure of the full-length TRPV2 channel by cryo-EM.
Huynh, K.W., Cohen, M.R., Jiang, J., Samanta, A., Lodowski, D.T., Zhou, Z.H., Moiseenkova-Bell, V.Y.(2016) Nat Commun 7: 11130-11130
- PubMed: 27021073
- DOI: https://doi.org/10.1038/ncomms11130
- Primary Citation of Related Structures:
5HI9 - PubMed Abstract:
Transient receptor potential (TRP) proteins form a superfamily Ca(2+)-permeable cation channels regulated by a range of chemical and physical stimuli. Structural analysis of a 'minimal' TRP vanilloid subtype 1 (TRPV1) elucidated a mechanism of channel activation by agonists through changes in its outer pore region. Though homologous to TRPV1, other TRPV channels (TRPV2-6) are insensitive to TRPV1 activators including heat and vanilloids. To further understand the structural basis of TRPV channel function, we determined the structure of full-length TRPV2 at ∼5 Å resolution by cryo-electron microscopy. Like TRPV1, TRPV2 contains two constrictions, one each in the pore-forming upper and lower gates. The agonist-free full-length TRPV2 has wider upper and lower gates compared with closed and agonist-activated TRPV1. We propose these newly revealed TRPV2 structural features contribute to diversity of TRPV channels.
Organizational Affiliation:
Department of Pharmacology, School of Medicine, Case Western Reserve University, 10900 Euclid Avenue, Wood Building, W151D, Cleveland, Ohio 44106, USA.