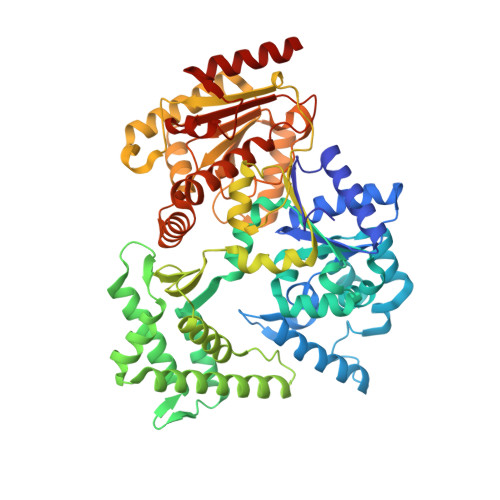
Mechanism of DNA loading by the DNA repair helicase XPD.
Constantinescu-Aruxandei, D., Petrovic-Stojanovska, B., Penedo, J.C., White, M.F., Naismith, J.H.(2016) Nucleic Acids Res 44: 2806-2815
- PubMed: 26896802
- DOI: https://doi.org/10.1093/nar/gkw102
- Primary Citation of Related Structures:
5H8C, 5H8W - PubMed Abstract:
The xeroderma pigmentosum group D (XPD) helicase is a component of the transcription factor IIH complex in eukaryotes and plays an essential role in DNA repair in the nucleotide excision repair pathway. XPD is a 5' to 3' helicase with an essential iron-sulfur cluster. Structural and biochemical studies of the monomeric archaeal XPD homologues have aided a mechanistic understanding of this important class of helicase, but several important questions remain open. In particular, the mechanism for DNA loading, which is assumed to require large protein conformational change, is not fully understood. Here, DNA binding by the archaeal XPD helicase from Thermoplasma acidophilum has been investigated using a combination of crystallography, cross-linking, modified substrates and biochemical assays. The data are consistent with an initial tight binding of ssDNA to helicase domain 2, followed by transient opening of the interface between the Arch and 4FeS domains, allowing access to a second binding site on helicase domain 1 that directs DNA through the pore. A crystal structure of XPD from Sulfolobus acidocaldiarius that lacks helicase domain 2 has an otherwise unperturbed structure, emphasizing the stability of the interface between the Arch and 4FeS domains in XPD.
Organizational Affiliation:
Biomedical Sciences Research Complex, University of St Andrews, Fife KY16 9ST, UK.