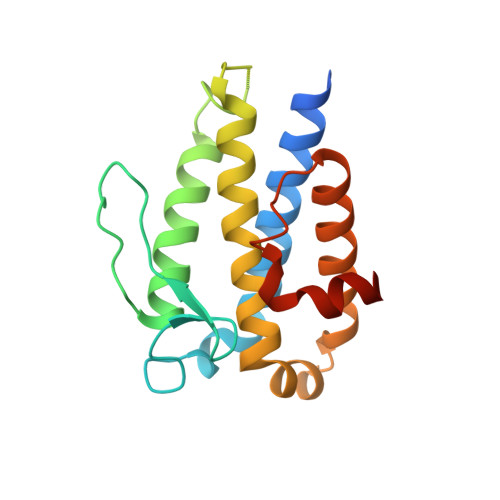
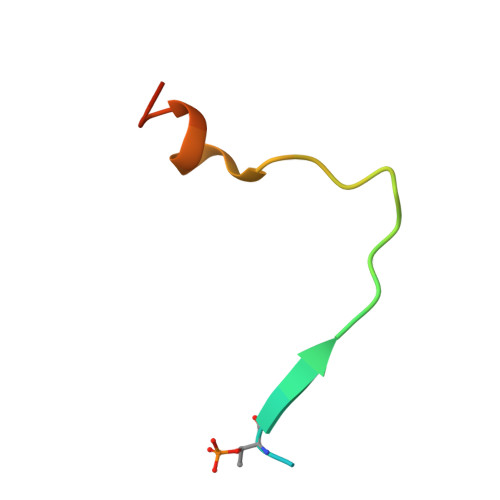
Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling.
Ni, L., Zheng, Y., Hara, M., Pan, D., Luo, X.(2015) Genes Dev 29: 1416-1431
- PubMed: 26108669
- DOI: https://doi.org/10.1101/gad.264929.115
- Primary Citation of Related Structures:
5BRK, 5BRM - PubMed Abstract:
The Mst-Lats kinase cascade is central to the Hippo tumor-suppressive pathway that controls organ size and tissue homeostasis. The adaptor protein Mob1 promotes Lats activation by Mst, but the mechanism remains unknown. Here, we show that human Mob1 binds to autophosphorylated docking motifs in active Mst2. This binding enables Mob1 phosphorylation by Mst2. Phosphorylated Mob1 undergoes conformational activation and binds to Lats1. We determine the crystal structures of phospho-Mst2-Mob1 and phospho-Mob1-Lats1 complexes, revealing the structural basis of both phosphorylation-dependent binding events. Further biochemical and functional analyses demonstrate that Mob1 mediates Lats1 activation through dynamic scaffolding and allosteric mechanisms. Thus, Mob1 acts as a phosphorylation-regulated coupler of kinase activation by virtue of its ability to engage multiple ligands. We propose that stepwise, phosphorylation-triggered docking interactions of nonkinase elements enhance the specificity and robustness of kinase signaling cascades.
Organizational Affiliation:
Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA;