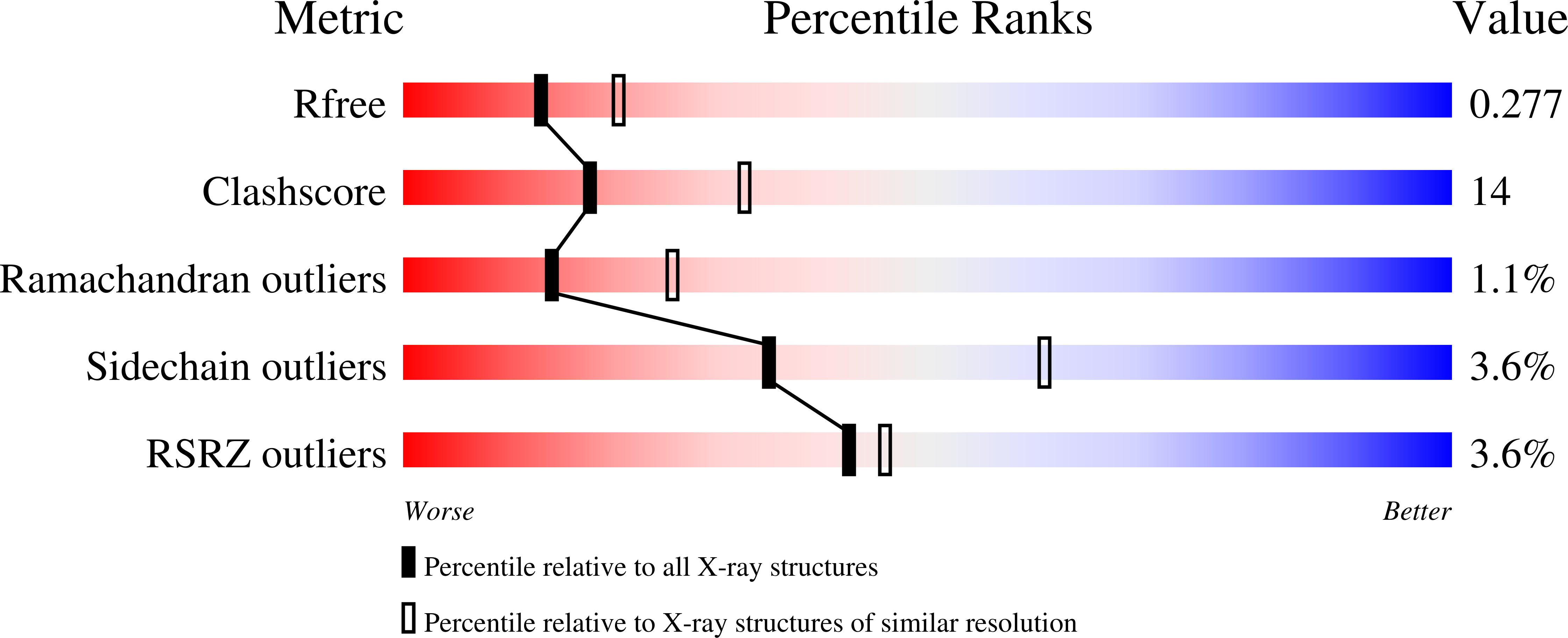
Re-Designing the Substrate Binding Pocket of Laccase for Enhanced Oxidation of Sinapic Acid
Pardo, I., Santiago, G., Gentili, P., Lucas, F., Monza, E., Medrano, F.J., Romero, A., Galli, C., Martinez, A.T., Guallar, V., Camarero, S.(2016) Catal Sci Technol 6: 3900