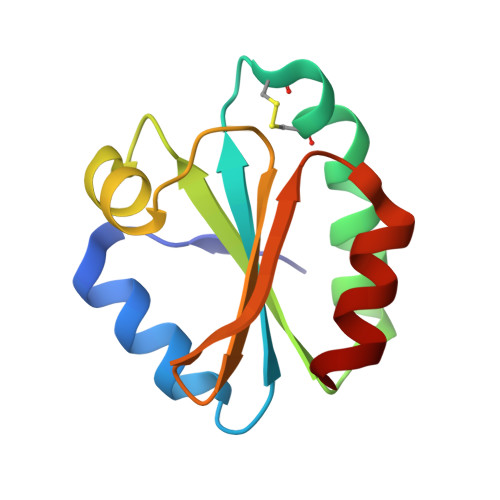
Crystallographic Studies Evidencing the High Energy Tolerance to Disrupting the Interface Disulfide Bond of Thioredoxin 1 from White Leg Shrimp Litopenaeus Vannamei.
Campos-Acevedo, A.A., Rudino-Pinera, E.(2014) Molecules 19: 21113
- PubMed: 25517346
- DOI: https://doi.org/10.3390/molecules191221113
- Primary Citation of Related Structures:
4V2L, 4V2M, 4V2N - PubMed Abstract:
Thioredoxin (Trx) is a small 12-kDa redox protein that catalyzes the reduction of disulfide bonds in proteins from different biological systems. A recent study of the crystal structure of white leg shrimp thioredoxin 1 from Litopenaeus vannamei (LvTrx) revealed a dimeric form of the protein mediated by a covalent link through a disulfide bond between Cys73 from each monomer. In the present study, X-ray-induced damage in the catalytic and the interface disulfide bond of LvTrx was studied at atomic resolution at different transmission energies of 8% and 27%, 12.8 keV at 100 K in the beamline I-24 at Diamond Light Source. We found that at an absorbed dose of 32 MGy, the X-ray induces the cleavage of the disulfide bond of each catalytic site; however, the interface disulfide bond was cleaved at an X-ray adsorbed dose of 85 MGy; despite being the most solvent-exposed disulfide bond in LvTrx (~50 Å2). This result clearly established that the interface disulfide bond is very stable and, therefore, less susceptible to being reduced by X-rays. In fact, these studies open the possibility of the existence in solution of a dimeric LvTrx.
Organizational Affiliation:
Departamento de Medicina molecular y Bioprocesos, Instituto de Biotecnología (IBT), Universidad Nacional Autónoma de México (UNAM), Avenida Universidad 2001, Colonia Chamilpa, Cuernavaca 62210, Mexico.