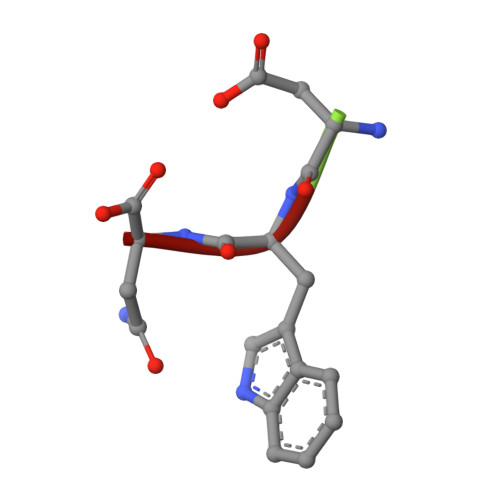
Inhibition by stabilization: targeting the Plasmodium falciparum aldolase-TRAP complex.
Nemetski, S.M., Cardozo, T.J., Bosch, G., Weltzer, R., O'Malley, K., Ejigiri, I., Kumar, K.A., Buscaglia, C.A., Nussenzweig, V., Sinnis, P., Levitskaya, J., Bosch, J.(2015) Malar J 14: 324-324
- PubMed: 26289816
- DOI: https://doi.org/10.1186/s12936-015-0834-9
- Primary Citation of Related Structures:
4TR9 - PubMed Abstract:
Emerging resistance of the malaria parasite Plasmodium to current therapies underscores the critical importance of exploring novel strategies for disease eradication. Plasmodium species are obligate intracellular protozoan parasites. They rely on an unusual form of substrate-dependent motility for their migration on and across host-cell membranes and for host cell invasion. This peculiar motility mechanism is driven by the 'glideosome', an actin-myosin associated, macromolecular complex anchored to the inner membrane complex of the parasite. Myosin A, actin, aldolase, and thrombospondin-related anonymous protein (TRAP) constitute the molecular core of the glideosome in the sporozoite, the mosquito stage that brings the infection into mammals. Virtual library screening of a large compound library against the PfAldolase-TRAP complex was used to identify candidate compounds that stabilize and prevent the disassembly of the glideosome. The mechanism of these compounds was confirmed by biochemical, biophysical and parasitological methods. A novel inhibitory effect on the parasite was achieved by stabilizing a protein-protein interaction within the glideosome components. Compound 24 disrupts the gliding and invasive capabilities of Plasmodium parasites in in vitro parasite assays. A high-resolution, ternary X-ray crystal structure of PfAldolase-TRAP in complex with compound 24 confirms the mode of interaction and serves as a platform for future ligand optimization. This proof-of-concept study presents a novel approach to anti-malarial drug discovery and design. By strengthening a protein-protein interaction within the parasite, an avenue towards inhibiting a previously "undruggable" target is revealed and the motility motor responsible for successful invasion of host cells is rendered inactive. This study provides new insights into the malaria parasite cell invasion machinery and convincingly demonstrates that liver cell invasion is dramatically reduced by 95 % in the presence of the small molecule stabilizer compound 24.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology, New York University School of Medicine, New York, USA. maureen.nemetski@gmail.com.