Structure and specificity of L-D-Transpeptidase from Mycobacterium tuberculosis and antibiotic resistance: Calcium binding promotes dimer formation
Gokulan, K., Khare, S., Cerniglia, C.E., Foley, S.L., Varughese, K.I.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| L,d-transpeptidase LdtB | 349 | Mycobacterium tuberculosis H37Rv | Mutation(s): 0 Gene Names: ldtB, Rv2518c, RVBD_2518c | 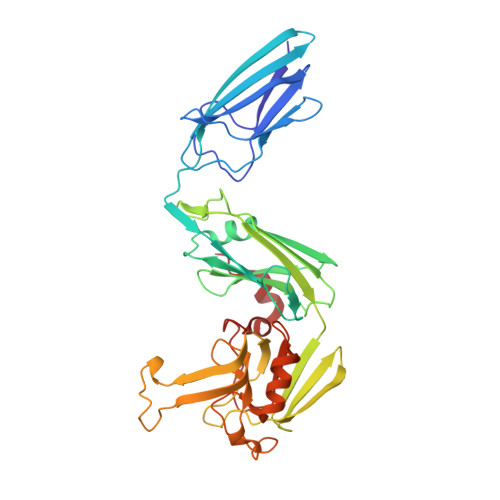 | |
UniProt | |||||
Find proteins for I6Y9J2 (Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)) Explore I6Y9J2 Go to UniProtKB: I6Y9J2 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | I6Y9J2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MLD Query on MLD | B [auth A] | GLCNAC(BETA1-4)-MURNAC(1,6-ANHYDRO)-L-ALA-GAMMA-D-GLU-MESO-A2PM-D-ALA C37 H59 N7 O20 UPFMKPIBAIPLHT-RSJSDIDPSA-N |  | ||
| 3V5 Query on 3V5 | C [auth A] | (3S,5S)-3-({[(aminomethyl)amino]methyl}sulfanyl)-5-[(2S)-1,3-dioxobutan-2-yl]-L-proline C11 H19 N3 O4 S SKDDBJCIWUNJNL-XKNYDFJKSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 57.272 | α = 90 |
| b = 65.881 | β = 90 |
| c = 207.456 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHENIX | model building |
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |