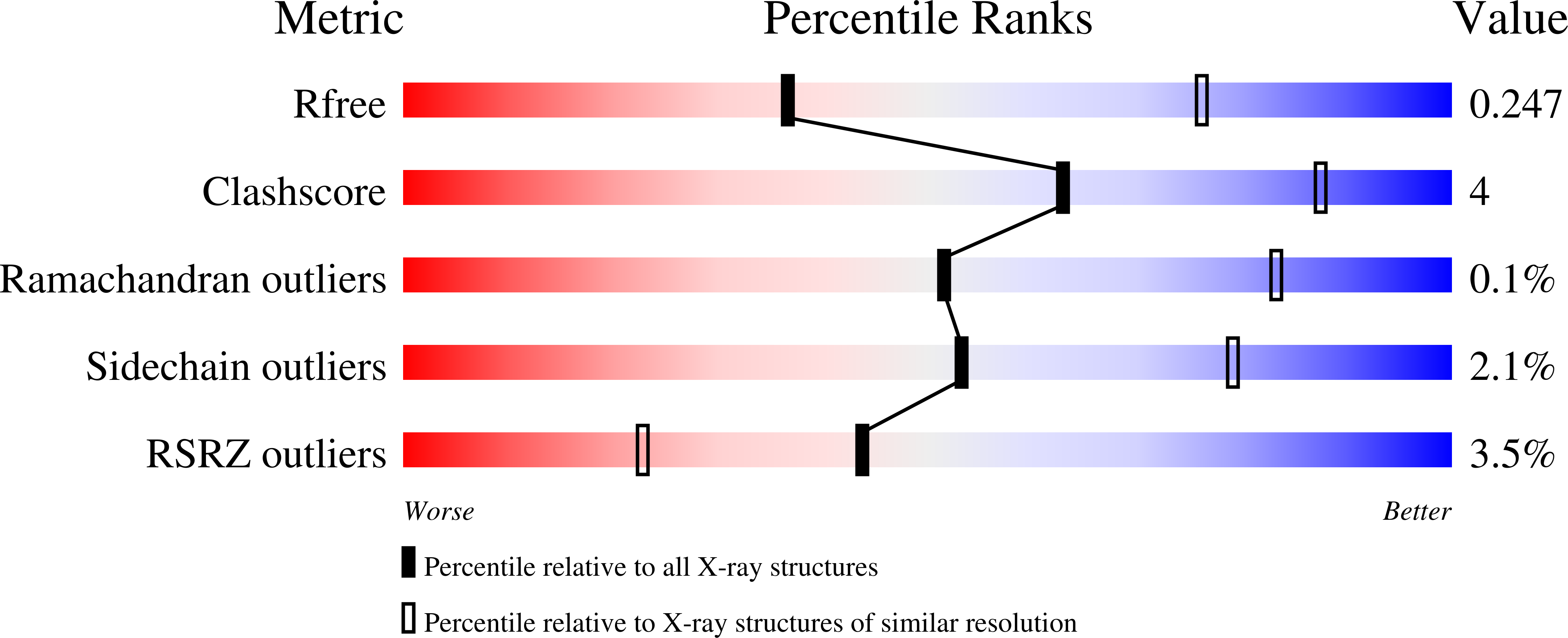
Structural basis for pore-forming mechanism of staphylococcal alpha-hemolysin.
Sugawara, T., Yamashita, D., Kato, K., Peng, Z., Ueda, J., Kaneko, J., Kamio, Y., Tanaka, Y., Yao, M.(2015) Toxicon 108: 226-231
- PubMed: 26428390
- DOI: https://doi.org/10.1016/j.toxicon.2015.09.033
- Primary Citation of Related Structures:
4P24, 4YHD - PubMed Abstract:
Staphylococcal α-hemolysin (α-HL) is a β-barrel pore-forming toxin (PFT) expressed by Staphylococcus aureus. α-HL is secreted as a water-soluble monomeric protein, which binds to target membranes and forms membrane-inserted heptameric pores. To explore the pore-forming mechanism of α-HL in detail, we determined the crystal structure of the α-HL monomer and prepore using H35A mutant and W179A/R200A mutant, respectively. Although the overall structure of the monomer was similar to that of other staphylococcal PFTs, a marked difference was observed in the N-terminal amino latch, which bent toward the prestem. Moreover, the prestem was fastened by the cap domain with a key hydrogen bond between Asp45 and Tyr118. Prepore structure showed that the transmembrane region is roughly formed with flexibility, although the upper half of the β-barrel is formed appropriately. Structure comparison among monomer, prepore and pore revealed a series of motions, in which the N-terminal amino latch released upon oligomerization destroys its own key hydrogen bond between Asp45-Tyr118. This action initiated the protrusion of the prestem. Y118F mutant and the N-terminal truncated mutant markedly decreased in the hemolytic activity, indicating the importance of the key hydrogen bond and the N-terminal amino latch on the pore formation. Based on these observations, we proposed a dynamic molecular mechanism of pore formation for α-HL.
Organizational Affiliation:
Graduate School of Life Science, Hokkaido University, Sapporo, 060-0810, Japan.