Non-invasive intravital imaging of cellular differentiation with a bright red-excitable fluorescent protein.
Chu, J., Haynes, R.D., Corbel, S.Y., Li, P., Gonzalez-Gonzalez, E., Burg, J.S., Ataie, N.J., Lam, A.J., Cranfill, P.J., Baird, M.A., Davidson, M.W., Ng, H.L., Garcia, K.C., Contag, C.H., Shen, K., Blau, H.M., Lin, M.Z.(2014) Nat Methods 11: 572-578
- PubMed: 24633408
- DOI: https://doi.org/10.1038/nmeth.2888
- Primary Citation of Related Structures:
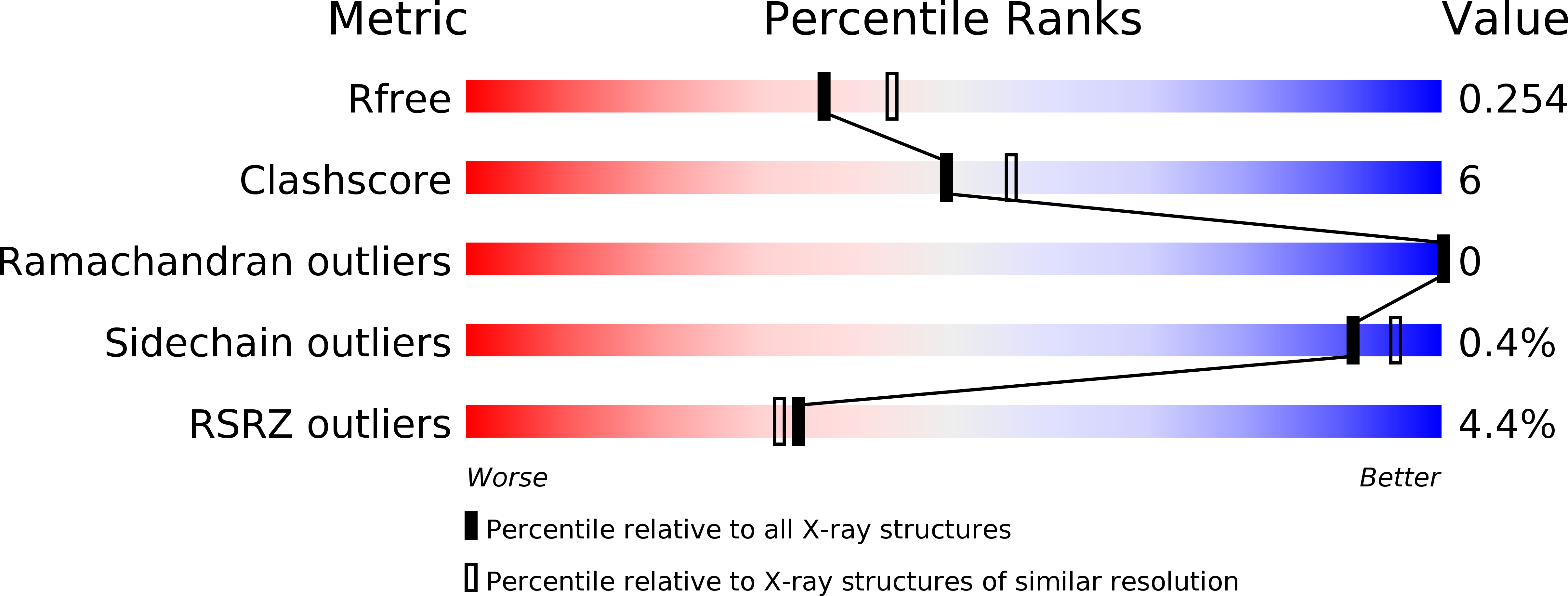
4OJ0, 4OQW - PubMed Abstract:
A method for non-invasive visualization of genetically labeled cells in animal disease models with micrometer-level resolution would greatly facilitate development of cell-based therapies. Imaging of fluorescent proteins (FPs) using red excitation light in the 'optical window' above 600 nm is one potential method for visualizing implanted cells. However, previous efforts to engineer FPs with peak excitation beyond 600 nm have resulted in undesirable reductions in brightness. Here we report three new red-excitable monomeric FPs obtained by structure-guided mutagenesis of mNeptune. Two of these, mNeptune2 and mNeptune2.5, demonstrate improved maturation and brighter fluorescence than mNeptune, whereas the third, mCardinal, has a red-shifted excitation spectrum without reduction in brightness. We show that mCardinal can be used to non-invasively and longitudinally visualize the differentiation of myoblasts into myocytes in living mice with high anatomical detail.
Organizational Affiliation:
1] Department of Bioengineering, Stanford University, Stanford, California, USA. [2] Department of Pediatrics, Stanford University School of Medicine, Stanford, California, USA.