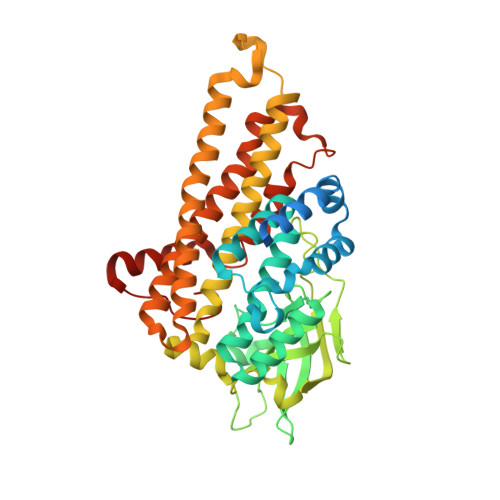
Structural insights into the stabilization of active, tetrameric DszC by its C-terminus.
Zhang, L., Duan, X., Zhou, D., Dong, Z., Ji, K., Meng, W., Li, G., Li, X., Yang, H., Ma, T., Rao, Z.(2014) Proteins 82: 2733-2743
- PubMed: 24975806
- DOI: https://doi.org/10.1002/prot.24638
- Primary Citation of Related Structures:
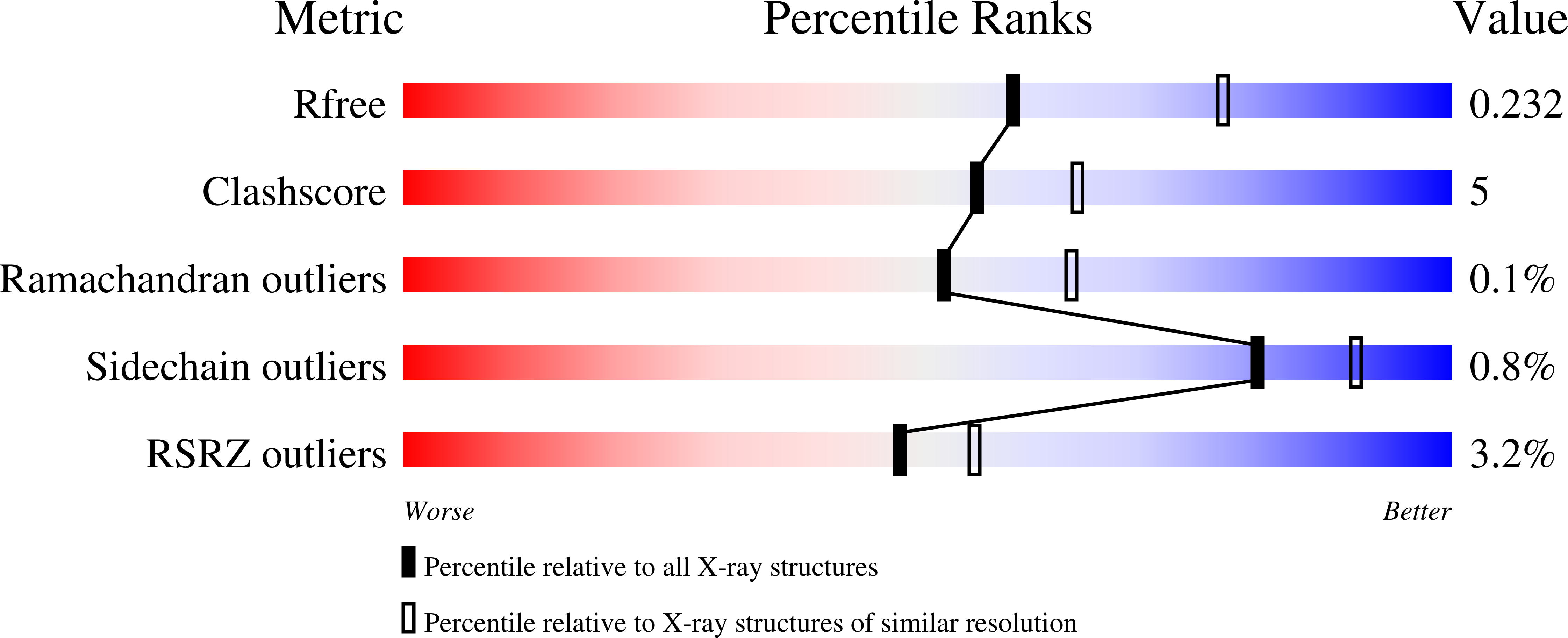
4NXL - PubMed Abstract:
Dibenzothiophene (DBT) is a typical sulfur-containing compound found in fossil fuels. This compound and its derivatives are resistant to the hydrodesulfurization method often used in industry, but they are susceptible to enzymatic desulfurization via the 4S pathway, which is a well-studied biochemical pathway consisting of four enzymes. DBT monooxygenase (DszC) from Rhodococcus erythropolis is involved in the first step of the 4S pathway. We determined the crystal structure of DszC, which reveals that, in contrast to several homologous proteins, the C-terminus (410-417) of DszC participates in the stabilization of the substrate-binding pocket. Analytical ultracentrifugation analysis and enzymatic assays confirmed that the C-terminus is important for the stabilization of the active conformation of the substrate-binding pocket and the tetrameric state. Therefore, the C-terminus of DszC plays a significant role in the catalytic activity of this enzyme.
Organizational Affiliation:
College of Life Sciences, Nankai University, Tianjin, China.