Sequence, biophysical, and structural analyses of the PstS lipoprotein (BB0215) from Borrelia burgdorferi reveal a likely binding component of an ABC-type phosphate transporter.
Brautigam, C.A., Ouyang, Z., Deka, R.K., Norgard, M.V.(2014) Protein Sci 23: 200-212
- PubMed: 24318969
- DOI: https://doi.org/10.1002/pro.2406
- Primary Citation of Related Structures:
4N13 - PubMed Abstract:
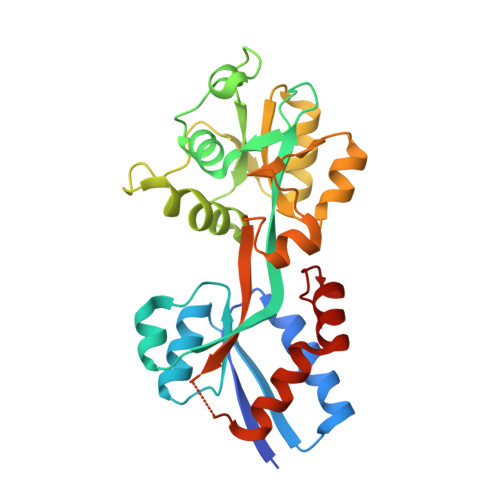
The Lyme disease agent Borrelia burgdorferi, which is transmitted via a tick vector, is dependent on its tick and mammalian hosts for a number of essential nutrients. Like other bacterial diderms, it must transport these biochemicals from the extracellular milieu across two membranes, ultimately to the B. burgdorferi cytoplasm. In the current study, we established that a gene cluster comprising genes bb0215 through bb0218 is cotranscribed and is therefore an operon. Sequence analysis of these proteins suggested that they are the components of an ABC-type transporter responsible for translocating phosphate anions from the B. burgdorferi periplasm to the cytoplasm. Biophysical experiments established that the putative ligand-binding protein of this system, BbPstS (BB0215), binds to phosphate in solution. We determined the high-resolution (1.3 Å) crystal structure of the protein in the absence of phosphate, revealing that the protein's fold is similar to other phosphate-binding proteins, and residues that are implicated in phosphate binding in other such proteins are conserved in BbPstS. Taken together, the gene products of bb0215-0218 function as a phosphate transporter for B. burgdorferi.
Organizational Affiliation:
Department of Biophysics, The University of Texas Southwestern Medical Center, Dallas, Texas, 75390.