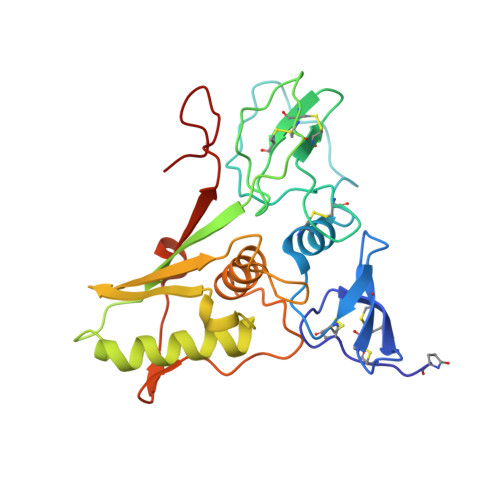
Crystal structure and its bearing towards an understanding of key biological functions of EpCAM.
Pavsic, M., Guncar, G., Djinovic-Carugo, K., Lenarcic, B.(2014) Nat Commun 5: 4764-4764
- PubMed: 25163760
- DOI: https://doi.org/10.1038/ncomms5764
- Primary Citation of Related Structures:
4MZV - PubMed Abstract:
EpCAM (epithelial cell adhesion molecule), a stem and carcinoma cell marker, is a cell surface protein involved in homotypic cell-cell adhesion via intercellular oligomerization and proliferative signalling via proteolytic cleavage. Despite its use as a diagnostic marker and being a drug target, structural details of this conserved vertebrate-exclusive protein remain unknown. Here we present the crystal structure of a heart-shaped dimer of the extracellular part of human EpCAM. The structure represents a cis-dimer that would form at cell surfaces and may provide the necessary structural foundation for the proposed EpCAM intercellular trans-tetramerization mediated by a membrane-distal region. By combining biochemical, biological and structural data on EpCAM, we show how proteolytic processing at various sites could influence structural integrity, oligomeric state and associated functionality of the molecule. We also describe the epitopes of this therapeutically important protein and explain the antigenicity of its regions.
Organizational Affiliation:
Department of Chemistry and Biochemistry, Faculty of Chemistry and Chemical Technology, University of Ljubljana, Večna pot 113, Ljubljana SI-1000, Slovenia.