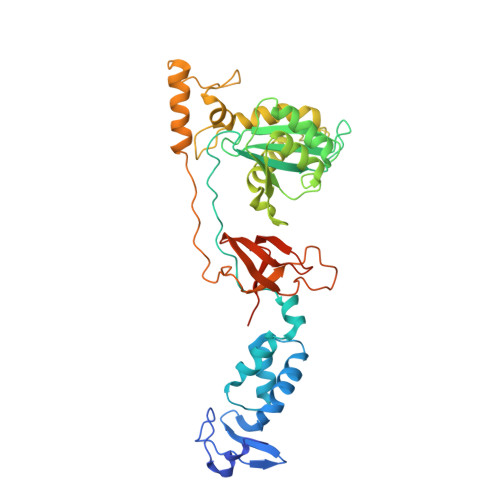
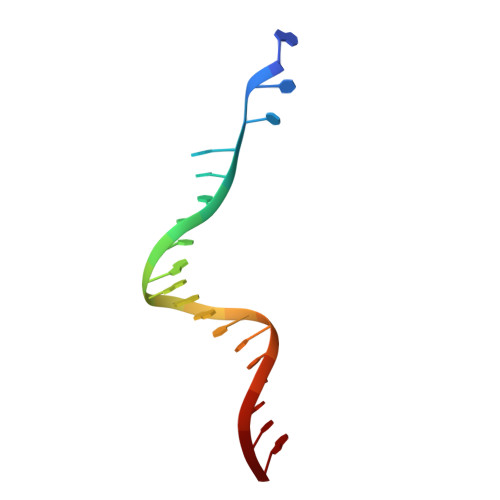
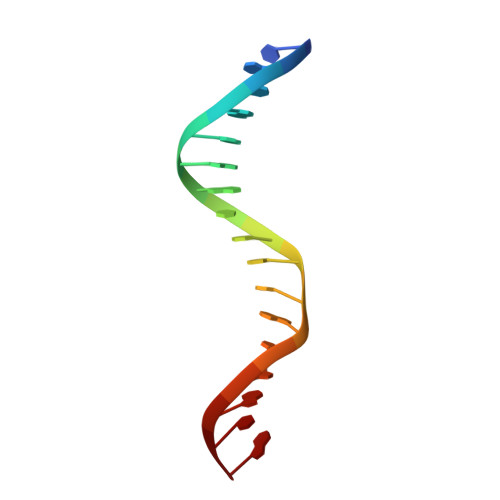
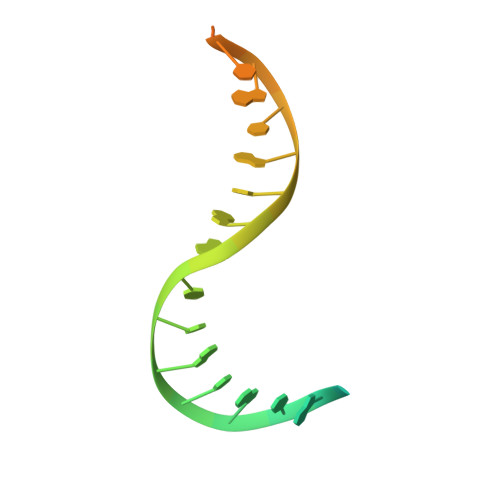
3'-Processing and strand transfer catalysed by retroviral integrase in crystallo.
Hare, S., Maertens, G.N., Cherepanov, P.(2012) EMBO J 468: 326-329
- PubMed: 22580823
- DOI: https://doi.org/10.1038/emboj.2012.118
- Primary Citation of Related Structures:
4E7H, 4E7I, 4E7J, 4E7K, 4E7L - PubMed Abstract:
Retroviral integrase (IN) is responsible for two consecutive reactions, which lead to insertion of a viral DNA copy into a host cell chromosome. Initially, the enzyme removes di- or trinucleotides from viral DNA ends to expose 3'-hydroxyls attached to the invariant CA dinucleotides (3'-processing reaction). Second, it inserts the processed 3'-viral DNA ends into host chromosomal DNA (strand transfer). Herein, we report a crystal structure of prototype foamy virus IN bound to viral DNA prior to 3'-processing. Furthermore, taking advantage of its dependence on divalent metal ion cofactors, we were able to freeze trap the viral enzyme in its ground states containing all the components necessary for 3'-processing or strand transfer. Our results shed light on the mechanics of retroviral DNA integration and explain why HIV IN strand transfer inhibitors are ineffective against the 3'-processing step of integration. The ground state structures moreover highlight a striking substrate mimicry utilized by the inhibitors in their binding to the IN active site and suggest ways to improve upon this clinically relevant class of small molecules.
Organizational Affiliation:
Division of Infectious Diseases, Imperial College London, London, UK.