Fluorescent proteins in Amphioxus have strickingly different brightness, yet only few (but key) molecular differences
Bomati, E.K., Haley, J.E., Noel, J.P., Deheyn, D.D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Amphioxus Green Fluorescent Protein, GFPc1a | 214 | Branchiostoma floridae | Mutation(s): 1 Gene Names: BRAFLDRAFT_75523 | 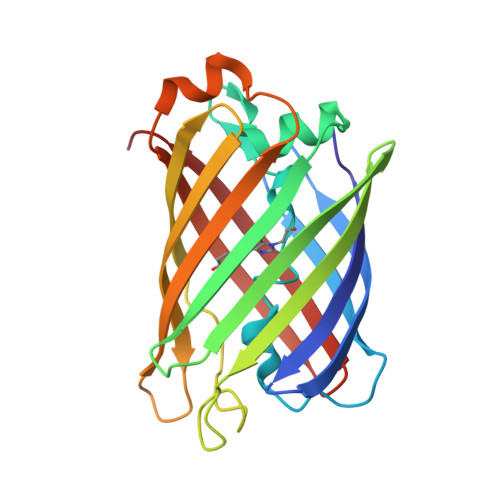 | |
UniProt | |||||
Find proteins for C3YRA3 (Branchiostoma floridae) Explore C3YRA3 Go to UniProtKB: C3YRA3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | C3YRA3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CR2 Query on CR2 | A, B, C, D, E A, B, C, D, E, F, G, H | L-PEPTIDE LINKING | C13 H13 N3 O4 |  | GLY, TYR, GLY |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 158.76 | α = 90 |
| b = 130.46 | β = 128.39 |
| c = 106.33 | γ = 90 |
| Software Name | Purpose |
|---|---|
| Blu-Ice | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |