Crystal Structure of the Shrimp Proliferating Cell Nuclear Antigen: Structural Complementarity with Wssv DNA Polymerase Pip-Box.
Carrasco-Miranda, J.S., Lopez-Zavala, A.A., Arvizu-Flores, A.A., Garcia-Orozco, K.D., Stojanoff, V., Rudino-Pinera, E., Brieba, L.G., Sotelo-Mundo, R.R.(2014) PLoS One 9: 94369
- PubMed: 24728082
- DOI: https://doi.org/10.1371/journal.pone.0094369
- Primary Citation of Related Structures:
4CS5 - PubMed Abstract:
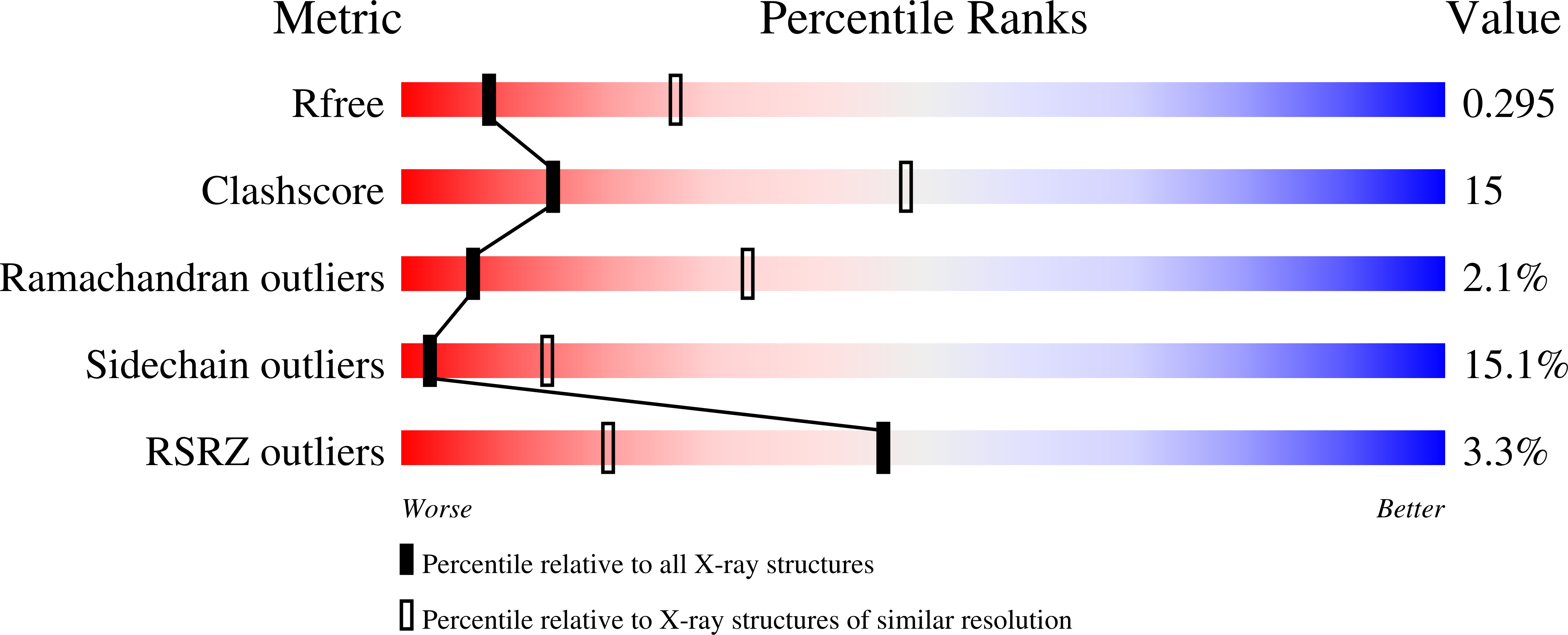
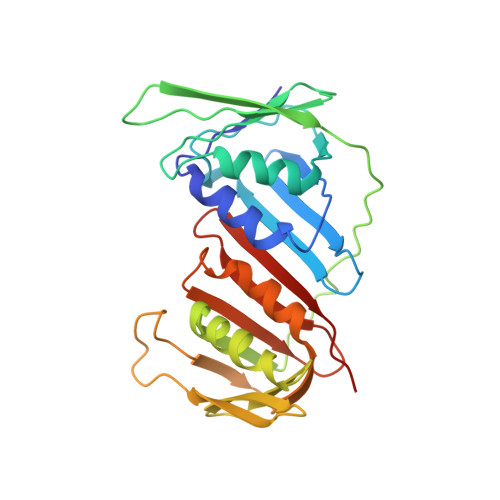
DNA replication requires processivity factors that allow replicative DNA polymerases to extend long stretches of DNA. Some DNA viruses encode their own replicative DNA polymerase, such as the white spot syndrome virus (WSSV) that infects decapod crustaceans but still require host replication accessory factors. We have determined by X-ray diffraction the three-dimensional structure of the Pacific white leg shrimp Litopenaeus vannamei Proliferating Cell Nuclear Antigen (LvPCNA). This protein is a member of the sliding clamp family of proteins, that binds DNA replication and DNA repair proteins through a motif called PIP-box (PCNA-Interacting Protein). The crystal structure of LvPCNA was refined to a resolution of 3 Å, and allowed us to determine the trimeric protein assembly and details of the interactions between PCNA and the DNA. To address the possible interaction between LvPCNA and the viral DNA polymerase, we docked a theoretical model of a PIP-box peptide from the WSSV DNA polymerase within LvPCNA crystal structure. The theoretical model depicts a feasible model of interaction between both proteins. The crystal structure of shrimp PCNA allows us to further understand the mechanisms of DNA replication processivity factors in non-model systems.
Organizational Affiliation:
Centro de Investigación en Alimentación y Desarrollo, A.C. Hermosillo, Sonora, México.