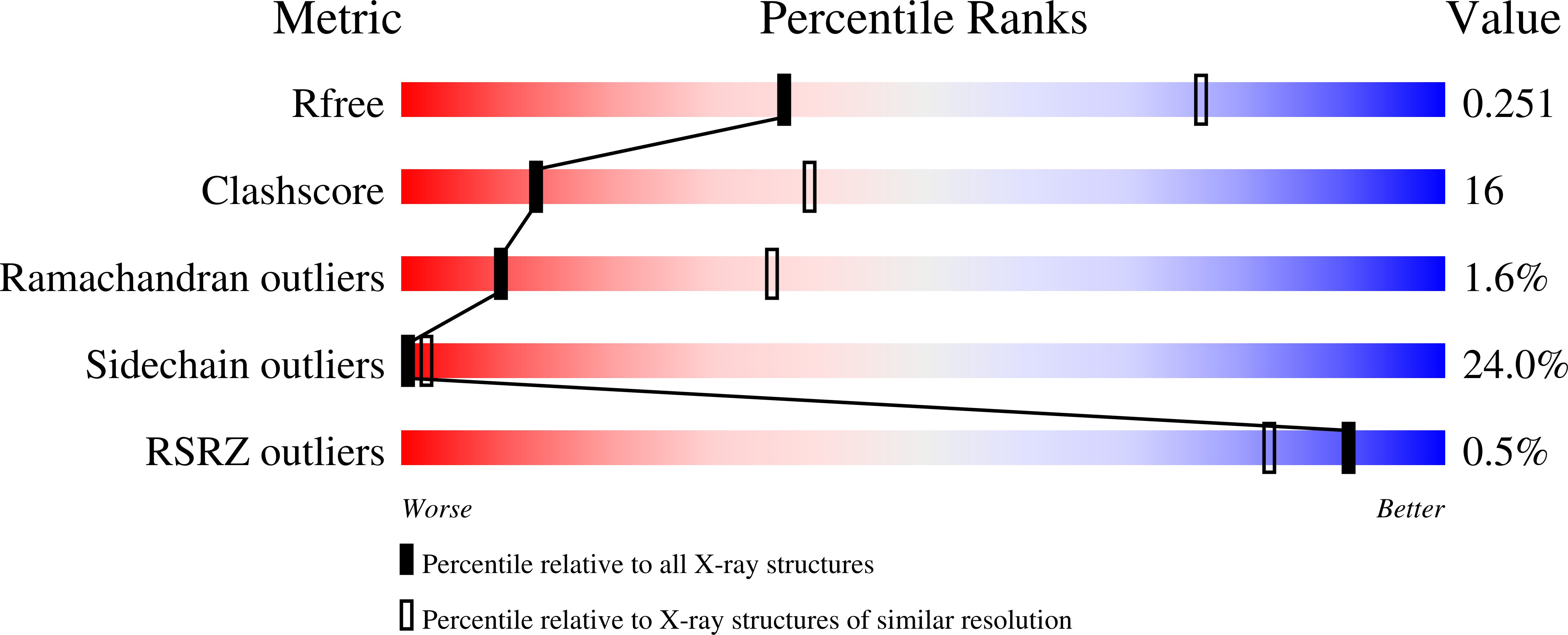
Solution and Crystallographic Structures of the Central Region of the Phosphoprotein from Human Metapneumovirus
Leyrat, C., Renner, M., Harlos, K., Grimes, J.M.(2013) PLoS One 8: 80371
- PubMed: 24224051
- DOI: https://doi.org/10.1371/journal.pone.0080371
- Primary Citation of Related Structures:
4BXT - PubMed Abstract:
Human metapneumovirus (HMPV) of the family Paramyxoviridae is a major cause of respiratory illness worldwide. Phosphoproteins (P) from Paramyxoviridae are essential co-factors of the viral RNA polymerase that form tetramers and possess long intrinsically disordered regions (IDRs). We located the central region of HMPV P (P(ced)) which is involved in tetramerization using disorder analysis and modeled its 3D structure ab initio using Rosetta fold-and-dock. We characterized the solution-structure of P(ced) using small angle X-ray scattering (SAXS) and carried out direct fitting to the scattering data to filter out incorrect models. Molecular dynamics simulations (MDS) and ensemble optimization were employed to select correct models and capture the dynamic character of P(ced). Our analysis revealed that oligomerization involves a compact central core located between residues 169-194 (P(core)), that is surrounded by flexible regions with α-helical propensity. We crystallized this fragment and solved its structure at 3.1 Å resolution by molecular replacement, using the folded core from our SAXS-validated ab initio model. The RMSD between modeled and experimental tetramers is as low as 0.9 Å, demonstrating the accuracy of the approach. A comparison of the structure of HMPV P to existing mononegavirales P(ced) structures suggests that P(ced) evolved under weak selective pressure. Finally, we discuss the advantages of using SAXS in combination with ab initio modeling and MDS to solve the structure of small, homo-oligomeric protein complexes.
Organizational Affiliation:
Division of Structural Biology, University of Oxford, Oxford, United Kingdom.