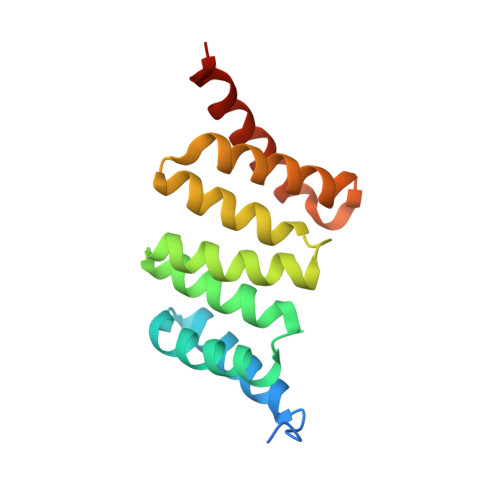
Crystal Structure of the Yersinia Enterocolitica Type III Secretion Chaperone Sycd in Complex with a Peptide of the Minor Translocator Yopd
Schreiner, M., Niemann, H.H.(2012) BMC Struct Biol 12: 12
- PubMed: 22708907
- DOI: https://doi.org/10.1186/1472-6807-12-13
- Primary Citation of Related Structures:
4AM9 - PubMed Abstract:
Type III secretion systems are used by Gram-negative bacteria as "macromolecular syringes" to inject effector proteins into eukaryotic cells. Two hydrophobic proteins called translocators form the necessary pore in the host cell membrane. Both translocators depend on binding to a single chaperone in the bacterial cytoplasm to ensure their stability and efficient transport through the secretion needle. It was suggested that the conserved chaperones bind the more divergent translocators via a hexapeptide motif that is found in both translocators and conserved between species. We crystallized a synthetic decapeptide from the Yersinia enterocolitica minor type III secretion translocator YopD bound to its cognate chaperone SycD and determined the complex structure at 2.5 Å resolution. The structure of peptide-bound SycD is almost identical to that of apo SycD with an all helical fold consisting of three tetratricopeptide repeats (TPRs) and an additional C-terminal helix. Peptide-bound SycD formed a kinked head-to-head dimer that had previously been observed for the apo form of SycD. The homodimer interface comprises both helices of the first tetratricopeptide repeat. The YopD peptide bound in extended conformation into a mainly hydrophobic groove on the concave side of SycD. TPRs 1 and 2 of SycD form three hydrophobic pockets that accommodated the conserved hydrophobic residues at position 1, 3 and 6 of the translocator hexapeptide sequence. Two tyrosines that are highly conserved among translocator chaperones contribute to the hydrophobic patches but also form hydrogen bonds to the peptide backbone. The interaction between SycD and YopD is very similar to the binding of the Pseudomonas minor translocator PopD to its chaperone PcrH and the Shigella major translocator IpaB to its chaperone IpgC. This confirms the prediction made by Kolbe and co-workers that a hexapeptide with hydrophobic residues at three positions is a conserved chaperone binding motif. Because the hydrophobic groove on the concave side of translocator chaperones is involved in binding of the major and the minor translocator, simultaneous binding of both translocators to a single type III secretion class II chaperone appears unlikely.
Organizational Affiliation:
Department of Chemistry, Bielefeld University, PO Box 10 01 31, 33501 Bielefeld, Germany.