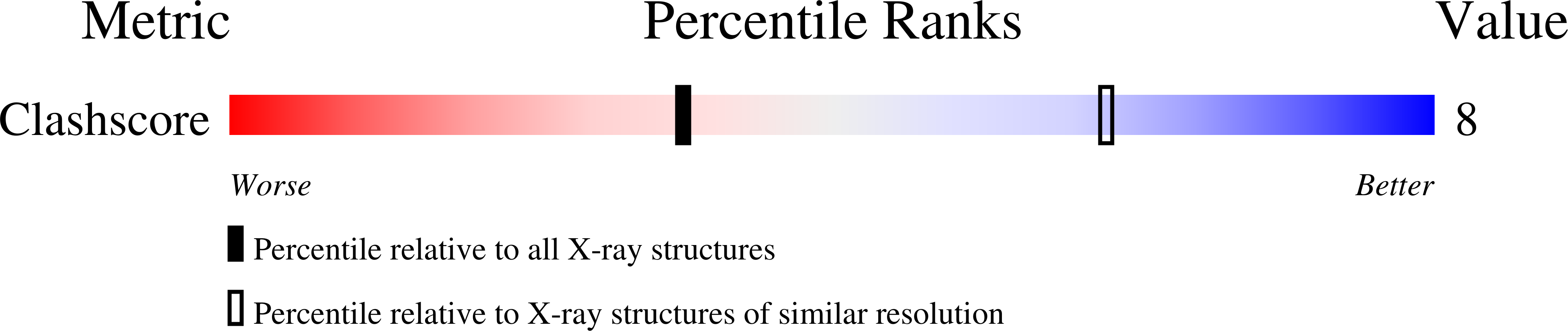
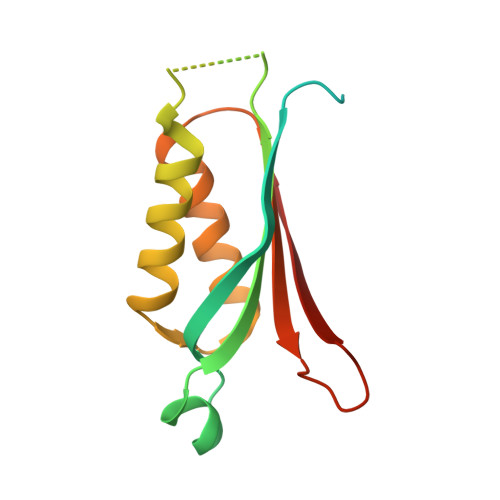
Folx from Pseudomonas Aeruginosa is Octameric in Both Crystal and Solution.
Gabrielsen, M., Beckham, K.S.H., Cogdell, R.J., Byron, O., Roe, A.J.(2012) FEBS Lett 586: 1160
- PubMed: 22575651
- DOI: https://doi.org/10.1016/j.febslet.2012.03.031
- Primary Citation of Related Structures:
4AEY - PubMed Abstract:
FolX encodes an epimerase that forms one step of the tetrahydrofolate biosynthetic pathway, which is of interest as it is an established target for important drugs. Here we report the crystal structure of FolX from the bacterial opportunistic pathogen Pseudomonas aeruginosa, as well as a detailed analysis of the protein in solution, using analytical ultracentrifugation (AUC) and small-angle X-ray scattering (SAXS). In combination, these techniques confirm that the protein is an octamer both in the crystal structure, and in solution.
Organizational Affiliation:
Institute of Infection, Immunity and Immunology, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK.