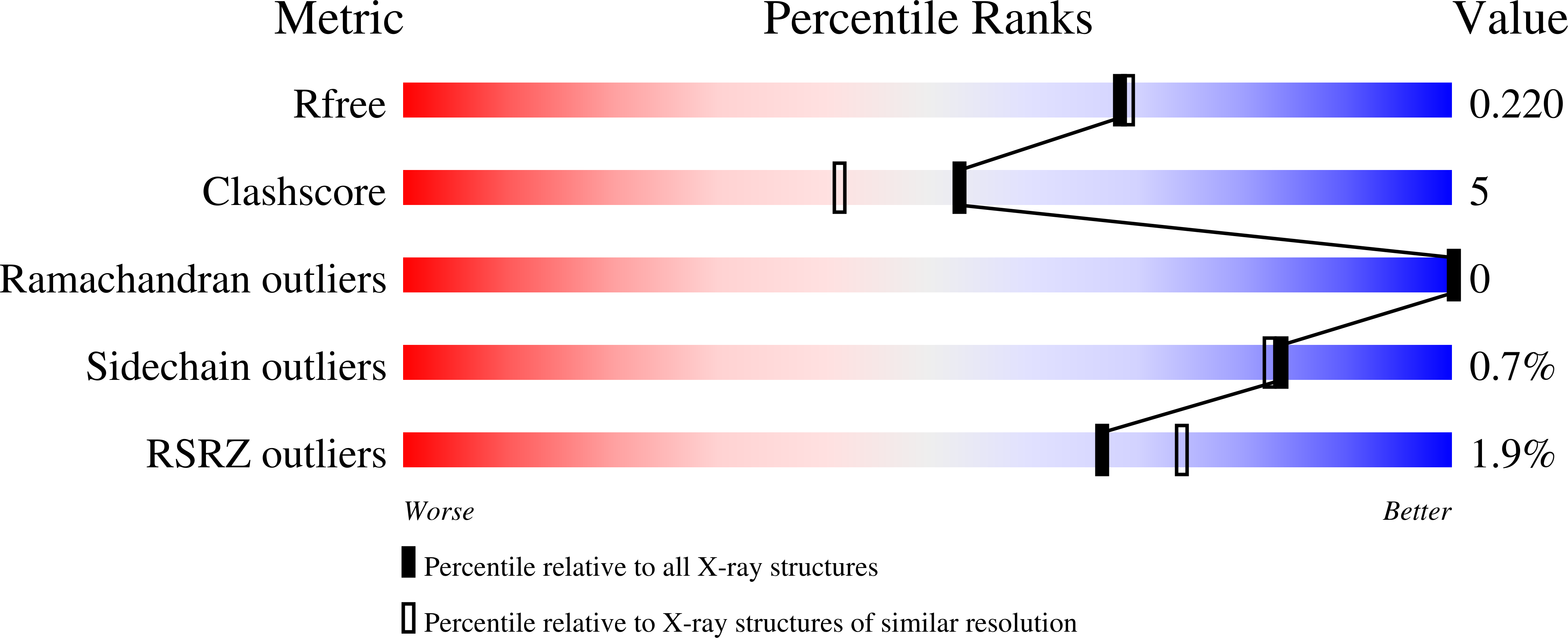
The soluble Y115E-Y117E variant of human glutaminyl cyclase is a valid target for X-ray and NMR screening of inhibitors against Alzheimer disease.
DiPisa, F., Pozzi, C., Benvenuti, M., Andreini, M., Marconi, G., Mangani, S.(2015) Acta Crystallogr F Struct Biol Commun 71: 986-992
- PubMed: 26249687
- DOI: https://doi.org/10.1107/S2053230X15010389
- Primary Citation of Related Structures:
4YU9, 4YWY - PubMed Abstract:
Recent developments in molecular pathology and genetics have allowed the identification of human glutaminyl cyclase (hQC) among the abnormal proteins involved in many neurodegenerative disorders. Difficulties in obtaining large quantities of pure protein may limit the use of crystallographic screening for drug development on this target. Site-directed mutagenesis experiments have led to the identification of some solvent-exposed residues that are absolutely critical to achieve increased solubility and to avoid precipitation of the enzyme in inclusion bodies when expressed in Escherichia coli. The designed variant Y115E-Y117E has been found to be able to provide large amounts of monodisperse, pure hQC from an E. coli expression system. To validate the use of the artificial construct as a target for large-scale X-ray and NMR screening campaigns in the search for new inhibitors of hQC, the X-ray crystal structures of the hQC Y115E-Y117E variant and of its adduct with the inhibitor PBD-150 were determined.
Organizational Affiliation:
Dipartimento di Biotecnologie, Chimica e Farmacia, Università di Siena, Via Aldo Moro 2, 53100 Siena, Italy.