Cleavage of amyloid precursor protein by an archaeal presenilin homologue PSH
Dang, S., Wu, S., Wang, J., Li, H., Huang, M., He, W., Li, Y.M., Wong, C.C., Shi, Y.(2015) Proc Natl Acad Sci U S A 112: 3344-3349
- PubMed: 25733893
- DOI: https://doi.org/10.1073/pnas.1502150112
- Primary Citation of Related Structures:
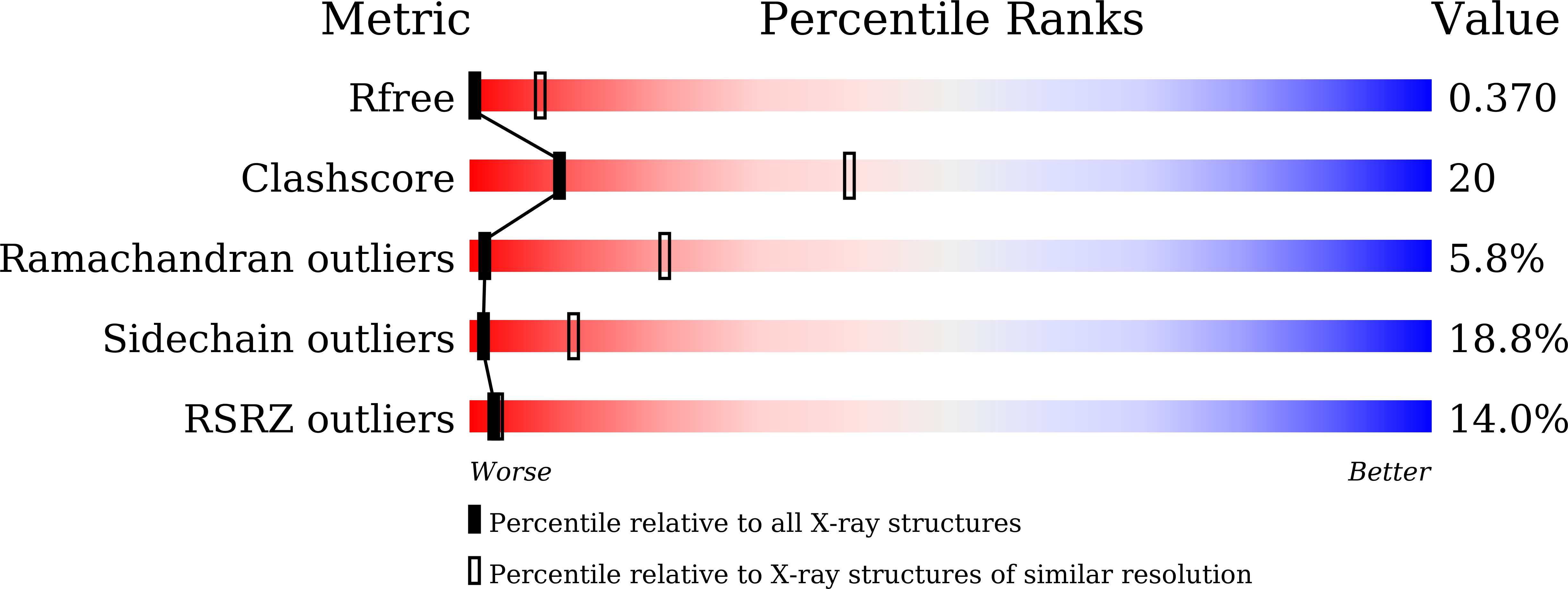
4Y6K - PubMed Abstract:
Aberrant cleavage of amyloid precursor protein (APP) by γ-secretase contributes to the development of Alzheimer's disease. More than 200 disease-derived mutations have been identified in presenilin (the catalytic subunit of γ-secretase), making modulation of γ-secretase activity a potentially attractive therapeutic opportunity. Unfortunately, the technical challenges in dealing with intact γ-secretase have hindered discovery of modulators and demand a convenient substitute approach. Here we report that, similar to γ-secretase, the archaeal presenilin homolog PSH faithfully processes the substrate APP C99 into Aβ42, Aβ40, and Aβ38. The molar ratio of the cleavage products Aβ42 over Aβ40 by PSH is nearly identical to that by γ-secretase. The proteolytic activity of PSH is specifically suppressed by presenilin-specific inhibitors. Known modulators of γ-secretase also modulate PSH similarly in terms of the Aβ42/Aβ40 ratio. Structural analysis reveals association of a known γ-secretase inhibitor with PSH between its two catalytic aspartate residues. These findings identify PSH as a surrogate protease for the screening of agents that may regulate the protease activity and the cleavage preference of γ-secretase.
Organizational Affiliation:
Ministry of Education Key Laboratory of Protein Science, Tsinghua-Peking Joint Center for Life Sciences, Center for Structural Biology, and.