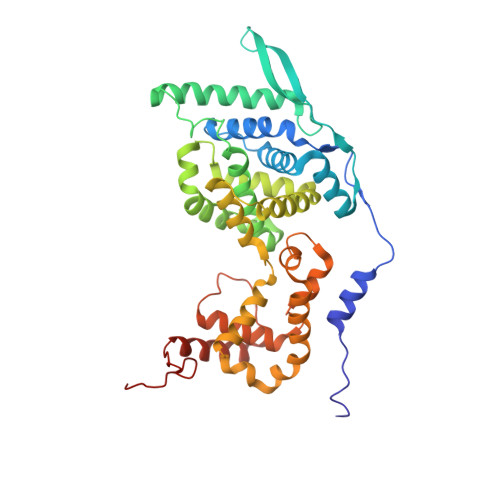
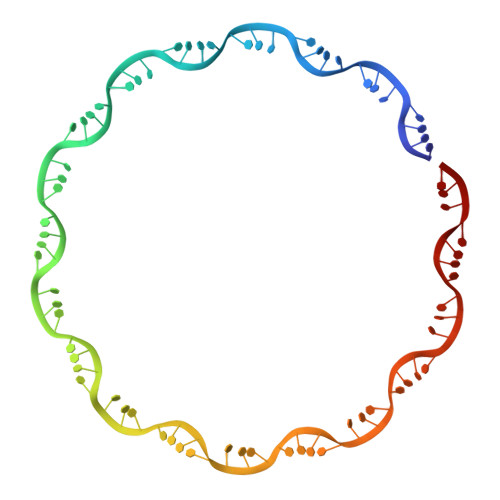
Structures of Respiratory Syncytial Virus Nucleocapsid Protein from Two Crystal Forms: Details of Potential Packing Interactions in the Native Helical Form.
El Omari, K., Dhaliwal, B., Ren, J., Abrescia, N.G.A., Lockyer, M., Powell, K.L., Hawkins, A.R., Stammers, D.K.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 1179
- PubMed: 22102022
- DOI: https://doi.org/10.1107/S1744309111029228
- Primary Citation of Related Structures:
2YHM, 4V5V - PubMed Abstract:
Respiratory syncytial virus (RSV) is a frequent cause of respiratory illness in infants, but there is currently no vaccine nor effective drug treatment against this virus. The RSV RNA genome is encapsidated and protected by a nucleocapsid protein; this RNA-nucleocapsid complex serves as a template for viral replication. Interest in the nucleocapsid protein has increased owing to its recent identification as the target site for novel anti-RSV compounds. The crystal structure of human respiratory syncytial virus nucleocapsid (HRSVN) was determined to 3.6 Å resolution from two crystal forms belonging to space groups P2(1)2(1)2(1) and P1, with one and four decameric rings per asymmetric unit, respectively. In contrast to a previous structure of HRSVN, the addition of phosphoprotein was not required to obtain diffraction-quality crystals. The HRSVN structures reported here, although similar to the recently published structure, present different molecular packing which may have some biological implications. The positions of the monomers are slightly shifted in the decamer, confirming the adaptability of the ring structure. The details of the inter-ring contacts in one crystal form revealed here suggest a basis for helical packing and that the stabilization of native HRSVN is via mainly ionic interactions.
Organizational Affiliation:
Division of Structural Biology and Oxford Protein Production Facility, The Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, England.