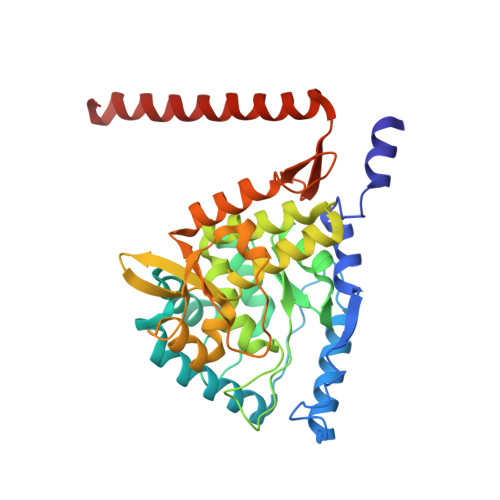
Crystal Structure of Human Tryptophane Hydroxylase 2 (Tph2), Catalytic Domain
Kopec, J., Oberholzer, A., Fitzpatrick, F., Newman, J., Tallant, C., Shrestha, L., Burgess-Brown, N., von Delft, F., Arrowsmith, C., Edwards, A., Bountra, C., Yue, W.W.To be published.