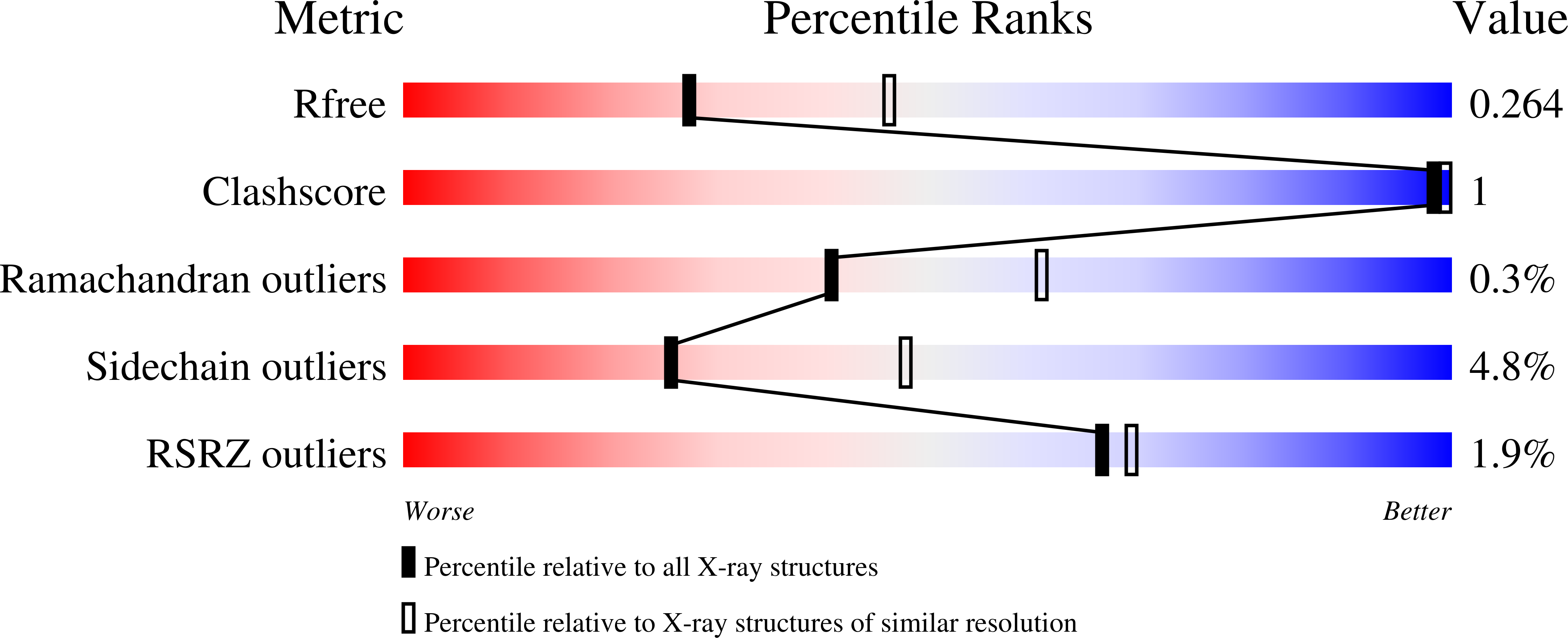
From Fragments to Leads: Novel Bacterial Nad+-Dependent DNA Ligase Inhibitors
Hale, M., Brassington, C., Carcanague, D., Embrey, K., Eyermann, C.J., Giacobbe, R.A., Gingipali, L., Gowravaram, M., Harang, J., Howard, T., Ioannidis, G., Jahic, H., Kutschke, A., Laganas, V.A., Loch, J., Miller, M.D., Murphy-Benenato, K.E., Oguto, H., Otterbein, L., Patel, S.J., Shapiro, A.B., Boriack-Sjodin, P.A.(2015) Tetrahedron Lett 56: 3108