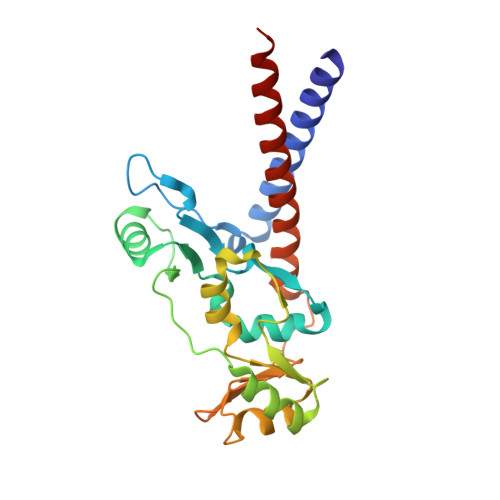
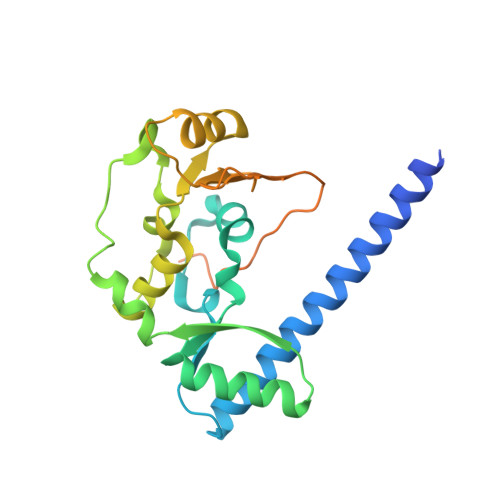
Structural basis for dimer information and DNA recognition of human SMC proteins
Uchiyama, S., Kawahara, K., Hosokawa, Y., Fukakusa, S., Oki, H., Nakamura, S., Noda, M., Takino, R., Miyahara, Y., Maruno, T., Kobayashi, Y., Ohkubo, T., Fukui, K.To be published.