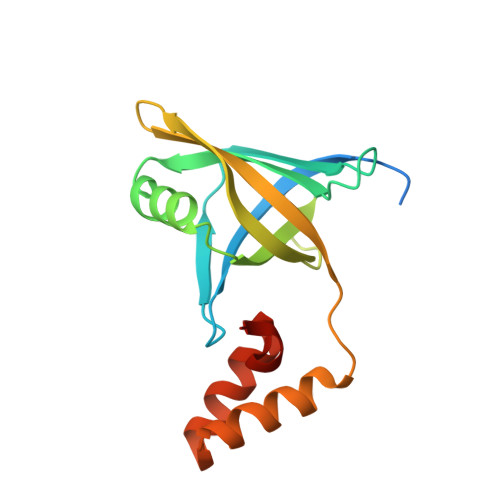
Crystal structure of YwpF from Staphylococcus aureus reveals its architecture comprised of a beta-barrel core domain resembling type VI secretion system proteins and a two-helix pair.
Lee, S.J., Lee, K.Y., Lee, K.Y., Kim, D.G., Kim, S.J., Lee, B.J.(2015) Proteins 83: 781-788
- PubMed: 25663006
- DOI: https://doi.org/10.1002/prot.24774
- Primary Citation of Related Structures:
4QXZ - PubMed Abstract:
The ywpF gene (SAV2097) of the Staphylococcus aureus strain Mu50 encodes the YwpF protein, which may play a role in antibiotic resistance. Here, we report the first crystal structure of the YwpF superfamily from S. aureus at 2.5-Å resolution. The YwpF structure consists of two regions: an N-terminal core β-barrel domain that shows structural similarity to type VI secretion system (T6SS) proteins (e.g., Hcp1, Hcp3, and EvpC) and a C-terminal two-helix pair. Although the monomer structure of S. aureus YwpF resembles those of T6SS proteins, the dimer/tetramer model of S. aureus YwpF is distinct from the functionally important hexameric ring of T6SS proteins. We therefore suggest that the S. aureus YwpF may have a different function compared to T6SS proteins.
Organizational Affiliation:
The Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Gwanak-Gu, Seoul, 151-742, Korea.