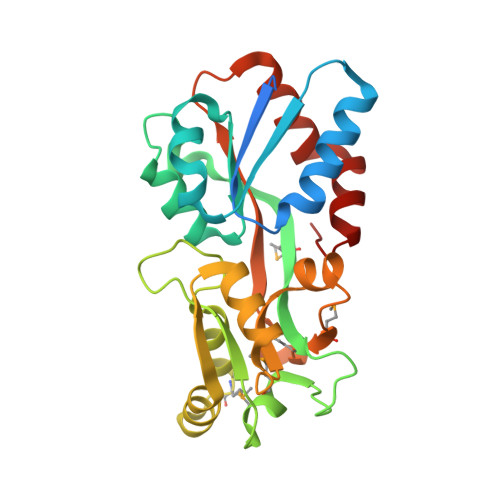
1.42 Angstrom resolution crystal structure of accessory colonization factor AcfC (acfC) in complex with D-aspartic acid
Halavaty, A.S., Wawrzak, Z., Dubrovska, I., Winsor, J., Minasov, G., Shuvalova, L., Filippova, E.V., Peterson, S.N., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.