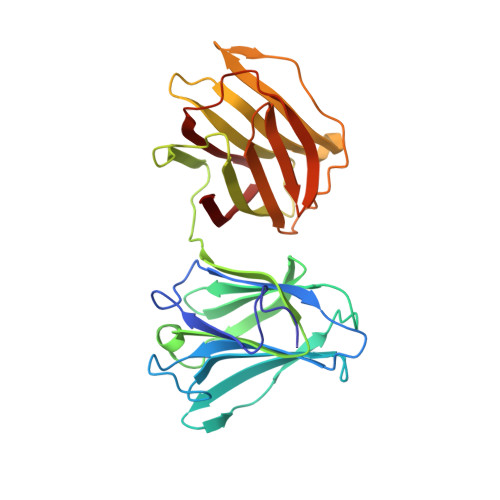
Structure of full-length Toxascaris leonina galectin with two carbohydrate-recognition domains.
Jeong, M.S., Hwang, H.G., Yu, H.S., Jang, S.B.(2013) Acta Crystallogr D Biol Crystallogr 69: 168-175
- PubMed: 23385453
- DOI: https://doi.org/10.1107/S0907444912045106
- Primary Citation of Related Structures:
4HL0 - PubMed Abstract:
The full-length crystal structure of Toxascaris leonine galectin (Tl-gal), a galectin-9 homologue protein, was determined at a resolution of 2.0 Å. Galectin-9 exhibits a variety of biological functions, including cell aggregation, eosinophil chemoattraction, activation and apoptosis of murine thymocytes, T cells and human melanoma cells. Similar to this galectin, Tl-gal may function as a regulatory molecule in the host immune system; however, no molecular or structural information has been reported for Tl-gal. Moreover, until now, there have been no reports of a full-length galectin structure. There are two molecules of Tl-gal per asymmetric unit in space group P2(1)2(1)2(1), and the N-terminal and C-terminal carbohydrate-recognition domains (NCRD and CCRD) of Tl-gal are composed of six-stranded β-sheets and five-stranded β-sheets with a short α-helix. The NCRD of Tl-gal resembles that of human galectin-7 and its CCRD resembles human galectin-9, but the residues in the interface and loop regions of the NCRD and CCRD are flexible and are related to interaction. Engagement of the T-cell immunoglobulin mucin-3 (Tim-3) immunoglobulin variable (IgV) domain by a galectin-9 ligand is known to be important for appropriate termination of T-helper 1 immune responses. To investigate the binding site of Tl-gal, the interaction between Tl-gal and Tim-3 was modelled. Tim-3 is docked into a major groove of the Tl-gal structure, which is larger and deeper than the minor groove. The structural information presented here will provide insight into the development of novel anti-inflammatory agents or selective modulators of immune response.
Organizational Affiliation:
Department of Molecular Biology, College of Natural Sciences, Pusan National University, Jangjeon-dong, Geumjeong-gu, Busan 609-735, Republic of Korea.