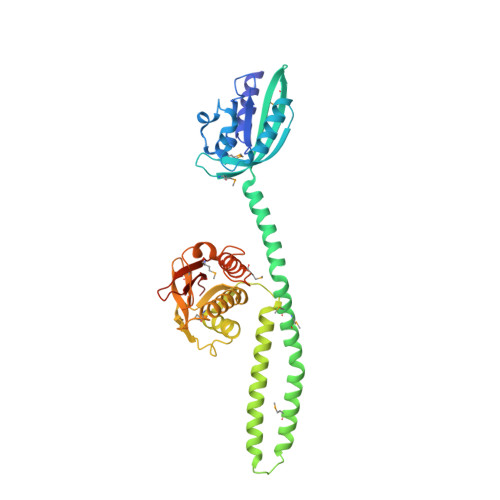
Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators.
Diensthuber, R.P., Bommer, M., Gleichmann, T., Moglich, A.(2013) Structure 21: 1127-1136
- PubMed: 23746806
- DOI: https://doi.org/10.1016/j.str.2013.04.024
- Primary Citation of Related Structures:
4GCZ - PubMed Abstract:
Two-component systems (TCSs), which comprise sensor histidine kinases (SHK) and response-regulator proteins, represent the predominant strategy by which prokaryotes sense and respond to a changing environment. Despite paramount biological importance, a dearth exists of intact SHK structures containing both sensor and effector modules. Here, we report the full-length crystal structure of the engineered, dimeric, blue-light-regulated SHK YF1 at 2.3 Å resolution, in which two N-terminal light-oxygen-voltage (LOV) photosensors are connected by a coiled coil to the C-terminal effector modules. A second coaxial coiled coil derived from the N-termini of the LOV photosensors and inserted between them crucially modulates light regulation: single mutations within this coiled coil attenuate or even invert the signal response of the TCS. Structural motifs identified in YF1 recur in signal receptors, and the underlying signaling principles and mechanisms may be widely shared between soluble and transmembrane, prokaryotic, and eukaryotic signal receptors of diverse biological activity.
Organizational Affiliation:
Biophysikalische Chemie, Institut für Biologie, Humboldt-Universität zu Berlin, 10115 Berlin, Germany. ralph.diensthuber@hu-berlin.de