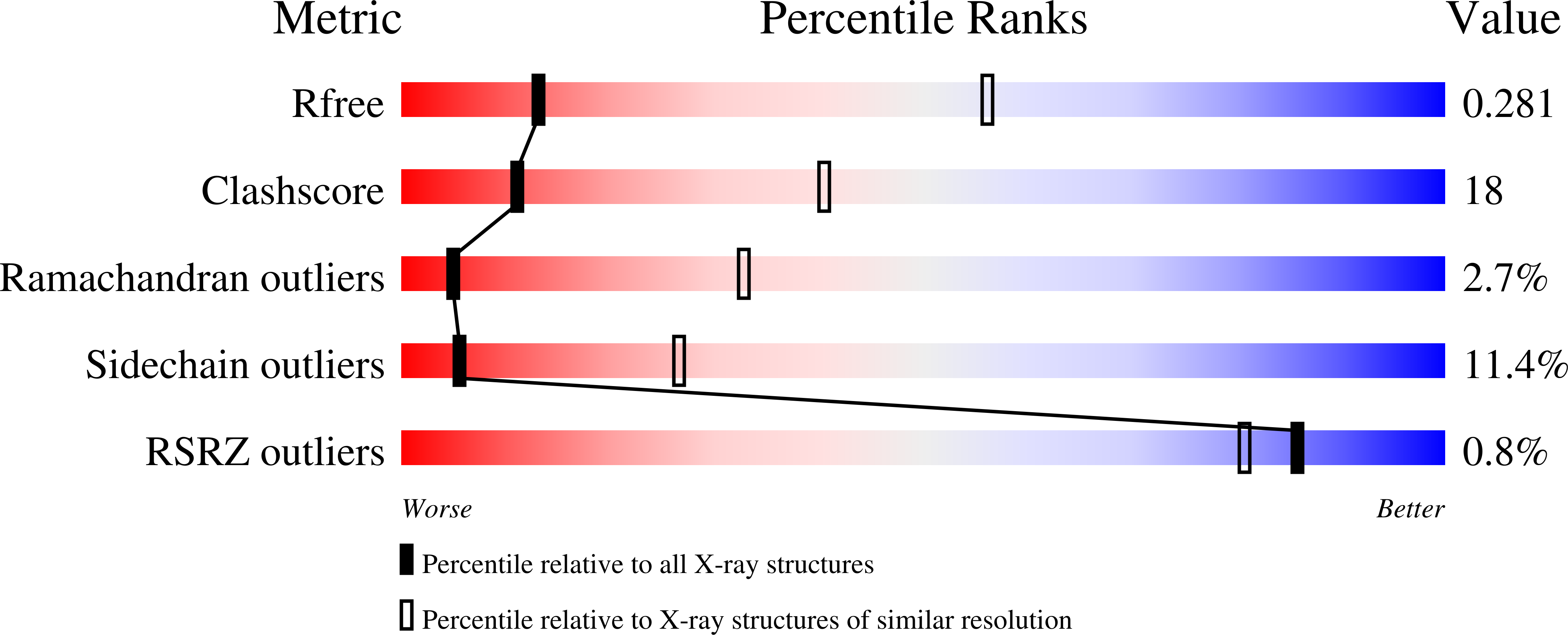
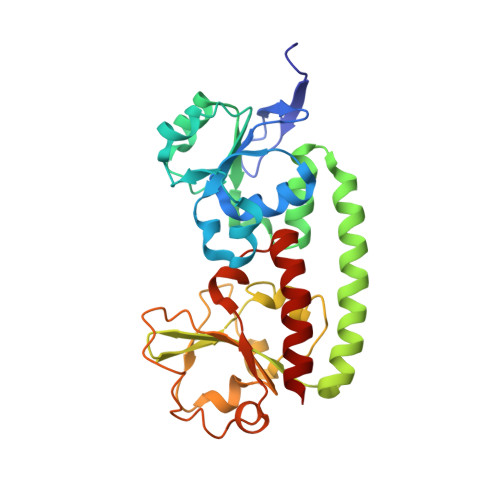
Crystal and solution structure analysis of FhuD2 from Staphylococcus aureus in multiple unliganded conformations and bound to ferrioxamine-B.
Podkowa, K.J., Briere, L.A., Heinrichs, D.E., Shilton, B.H.(2014) Biochemistry 53: 2017-2031
- PubMed: 24606332
- DOI: https://doi.org/10.1021/bi401349d
- Primary Citation of Related Structures:
4FIL, 4FKM, 4FNA - PubMed Abstract:
Iron acquisition is a central process for virtually all organisms. In Staphylococcus aureus, FhuD2 is a lipoprotein that is a high-affinity receptor for iron-bound hydroxamate siderophores. In this study, FhuD2 was crystallized bound to ferrioxamine-B (FXB), and also in its ligand-free state; the latter structures are the first for hydroxamate-binding receptors within this protein family. The structure of the FhuD2-FXB conformation shows that residues W197 and R199 from the C-terminal domain donate hydrogen bonds to the hydroxamate oxygens, and a ring of aromatic residues cradles the aliphatic arms connecting the hydroxamate moieties of the siderophore. The available ligand-bound structures of FhuD from Escherichia coli and YfiY from Bacillus cereus show that, despite a high degree of structural conservation, three protein families have evolved with critical siderophore binding residues on either the C-terminal domain (S. aureus), the N-terminal domain (E. coli), or both (B. cereus). Unliganded FhuD2 was crystallized in five conformations related by rigid body movements of the N- and C-terminal domains. Small-angle X-ray scattering (SAXS) indicates that the solution conformation of unliganded FhuD2 is more compact than the conformations observed in crystals. The ligand-induced conformational changes for FhuD2 in solution are relatively modest and depend on the identity of the siderophore. The crystallographic and SAXS results are used to discuss roles for the liganded and unliganded forms of FhuD2 in the siderophore transport mechanism.
Organizational Affiliation:
Department of Biochemistry and ‡Department of Microbiology and Immunology, The University of Western Ontario , London, Ontario, Canada N6A 5C1.