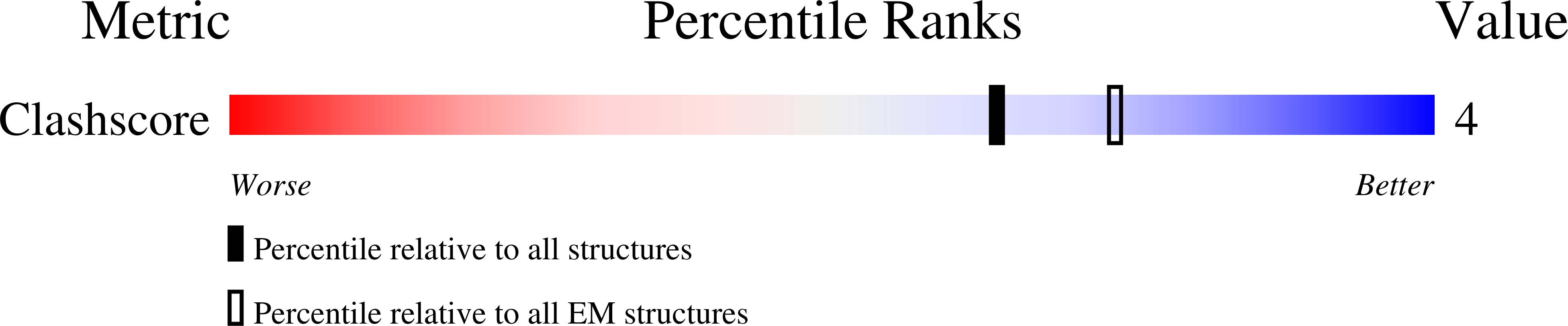Structure of the Immature Retroviral Capsid at 8A Resolution by Cryo-Electron Microscopy.
Bharat, T.A.M., Davey, N.E., Ulbrich, P., Riches, J.D., Marco, A.D., Rumlova, M., Sachse, C., Ruml, T., Briggs, J.A.G.(2012) Nature 487: 385
- PubMed: 22722831
- DOI: https://doi.org/10.1038/nature11169
- Primary Citation of Related Structures:
4ARD, 4ARG - PubMed Abstract:
The assembly of retroviruses such as HIV-1 is driven by oligomerization of their major structural protein, Gag. Gag is a multidomain polyprotein including three conserved folded domains: MA (matrix), CA (capsid) and NC (nucleocapsid). Assembly of an infectious virion proceeds in two stages. In the first stage, Gag oligomerization into a hexameric protein lattice leads to the formation of an incomplete, roughly spherical protein shell that buds through the plasma membrane of the infected cell to release an enveloped immature virus particle. In the second stage, cleavage of Gag by the viral protease leads to rearrangement of the particle interior, converting the non-infectious immature virus particle into a mature infectious virion. The immature Gag shell acts as the pivotal intermediate in assembly and is a potential target for anti-retroviral drugs both in inhibiting virus assembly and in disrupting virus maturation. However, detailed structural information on the immature Gag shell has not previously been available. For this reason it is unclear what protein conformations and interfaces mediate the interactions between domains and therefore the assembly of retrovirus particles, and what structural transitions are associated with retrovirus maturation. Here we solve the structure of the immature retroviral Gag shell from Mason-Pfizer monkey virus by combining cryo-electron microscopy and tomography. The 8-Å resolution structure permits the derivation of a pseudo-atomic model of CA in the immature retrovirus, which defines the protein interfaces mediating retrovirus assembly. We show that transition of an immature retrovirus into its mature infectious form involves marked rotations and translations of CA domains, that the roles of the amino-terminal and carboxy-terminal domains of CA in assembling the immature and mature hexameric lattices are exchanged, and that the CA interactions that stabilize the immature and mature viruses are almost completely distinct.
Organizational Affiliation:
Structural and Computational Biology Unit, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany.