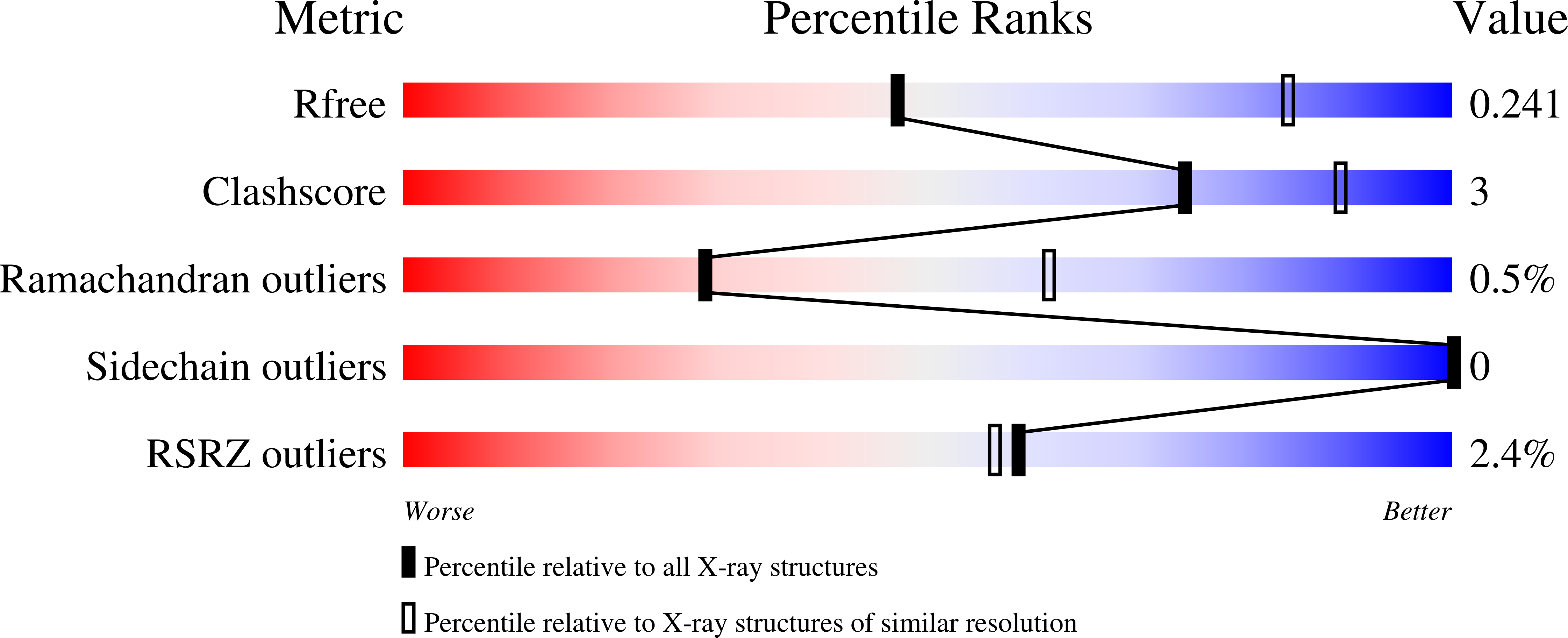
Heteroresistance to Fosfomycin is Predominant in Streptococcus Pneumoniae and Depends on Mura1 Gene.
Engel, H., Gutierrez-Fernandez, J., Fluckiger, C., Martinez-Ripoll, M., Muhlemann, K., Hermoso, J.A., Hilty, M., Hathaway, L.J.(2013) Antimicrob Agents Chemother 57: 2801
- PubMed: 23571543
- DOI: https://doi.org/10.1128/AAC.00223-13
- Primary Citation of Related Structures:
3ZH3, 3ZH4 - PubMed Abstract:
Fosfomycin targets the first step of peptidoglycan biosynthesis in Streptococcus pneumoniae catalyzed by UDP-N-acetylglucosamine enolpyruvyltransferase (MurA1). We investigated whether heteroresistance to fosfomycin occurs in S. pneumoniae. We found that of 11 strains tested, all but 1 (Hungary(19A)) displayed heteroresistance and that deletion of murA1 abolished heteroresistance. Hungary(19A) differs from the other strains by a single amino acid substitution in MurA1 (Ala(364)Thr). To test whether this substitution is responsible for the lack of heteroresistance, it was introduced into strain D39. The heteroresistance phenotype of strain D39 was not changed. Furthermore, no relevant structural differences between the MurA1 crystal structures of heteroresistant strain D39 and nonheteroresistant strain Hungary(19A) were found. Our results reveal that heteroresistance to fosfomycin is the predominant phenotype of S. pneumoniae and that MurA1 is required for heteroresistance to fosfomycin but is not the only factor involved. The findings provide a caveat for any future use of fosfomycin in the treatment of pneumococcal infections.
Organizational Affiliation:
Institute for Infectious Diseases, Faculty of Medicine, University of Bern, Bern, Switzerland.