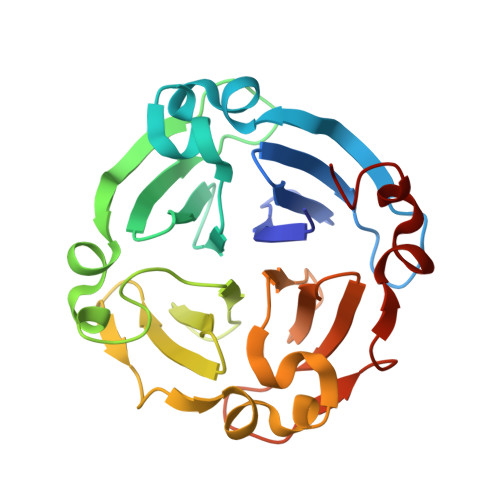
Crystal structure of a plant albumin from Cicer arietinum (chickpea) possessing hemopexin fold and hemagglutination activity
Sharma, U., Katre, U.V., Suresh, C.G.(2015) Planta
- PubMed: 25559942
- DOI: https://doi.org/10.1007/s00425-014-2236-6
- Primary Citation of Related Structures:
3S18, 3V6N - PubMed Abstract:
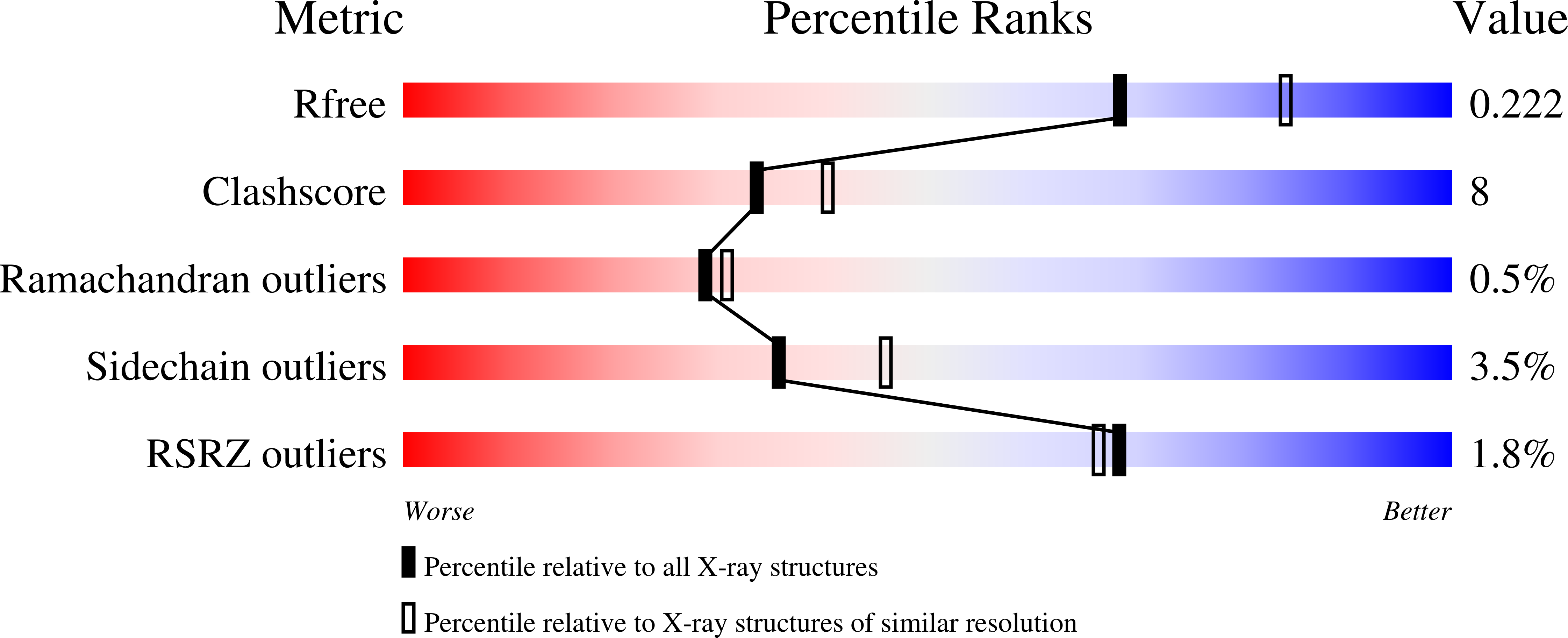
Crystal structure of a reported PA2 albumin from Cicer arietinum shows that it belongs to hemopexin fold family, has four beta-propeller motifs and possesses hemagglutination activity, making it different from known legume lectins. A plant albumin (PA2) from Cicer arietinum, presumably a lectin (CAL) owing to its hemagglutination activity which is inhibited by complex sugars as well as glycoproteins such as fetuin, desialylated fetuin and fibrinogen. The three-dimensional structure of this homodimeric protein has been determined using X-ray crystallography at 2.2 Å in two crystal forms: orthorhombic (P21212) and trigonal (P3). The structure determined using molecular replacement method and refined in orthorhombic crystal form reached R-factors R free 22.6 % and R work 18.2 % and in trigonal form had 22.3 and 17.9 % in the resolution range of 20.0-2.2 and 35.3-2.2 Å, respectively. Interestingly, unlike the known legume lectin fold, the structure of this homodimeric hemagglutinin belonged to hemopexin fold that consisted of four-bladed β-propeller architecture. Each subunit has a central cavity forming a channel, inside of which is lined with hydrophobic residues. The channel also bears binding sites for ligands such as calcium, sodium and chloride ions, iodine atom in the case of iodine derivative and water molecules. However, none of these ligands seem important for the sugar recognition. No monosaccharide sugar specificity could be detected using hemagglutination inhibition. Chemical modification studies identified a potential sugar-binding site per subunit molecule. Comparison of C-alpha atom positions in subunit structures showed that the deviations between the two crystal forms were more with respect to blades I and IV. Differences also existed between subunits in two forms in terms of type and site of ligand binding.
Organizational Affiliation:
Division of Biochemical Sciences, CSIR-National Chemical Laboratory, Pune, 411008, India.