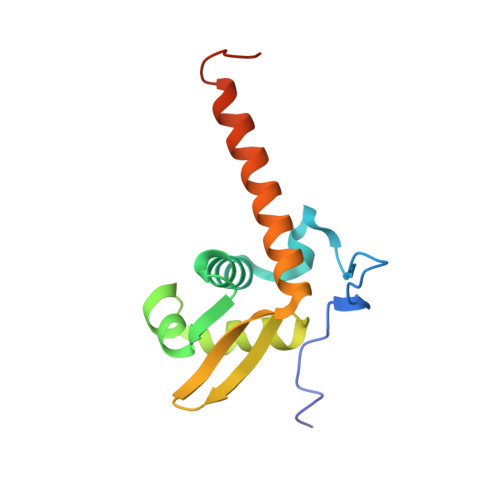
A reported archaeal mechanosensitive channel is a structural homolog of MarR-like transcriptional regulators.
Liu, Z., Walton, T.A., Rees, D.C.(2010) Protein Sci 19: 808-814
- PubMed: 20162616
- DOI: https://doi.org/10.1002/pro.360
- Primary Citation of Related Structures:
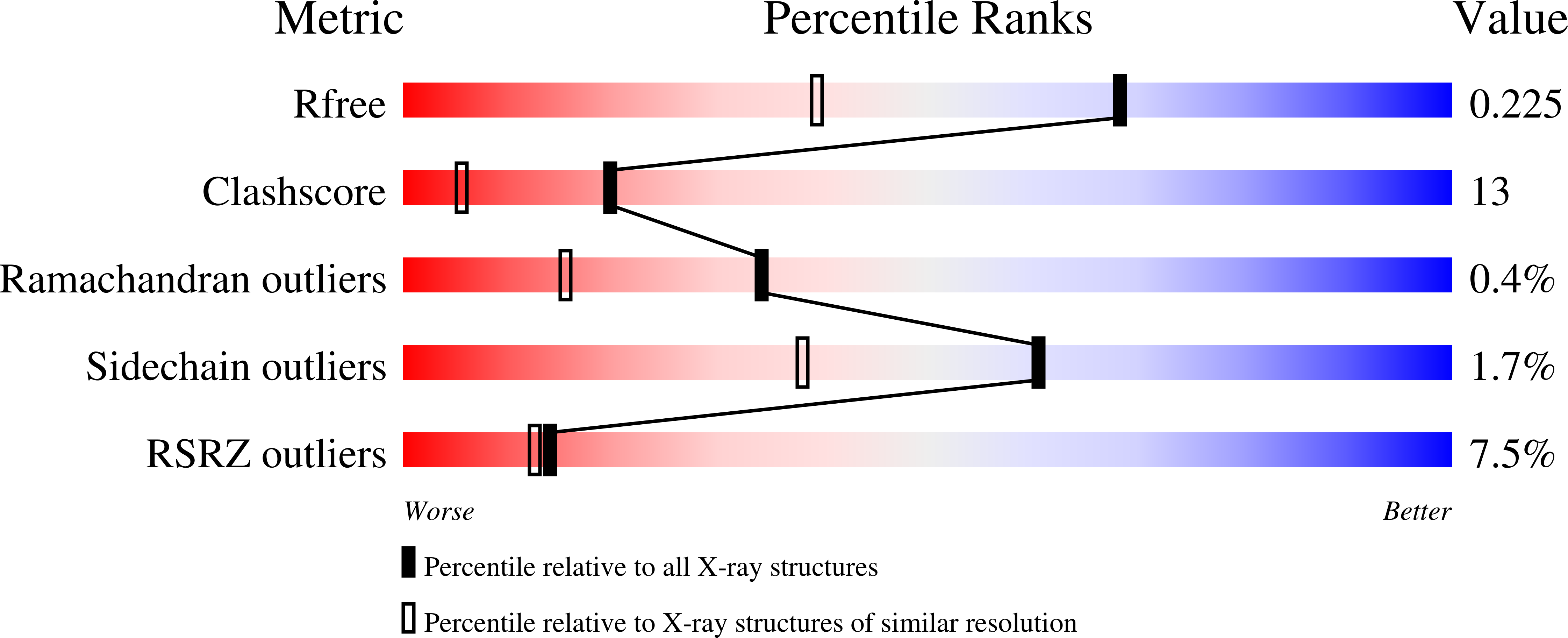
3LFK - PubMed Abstract:
Several archaeal mechanosensitive (MS) channels have been reported, including one from Thermoplasma volcanium designated MscTV. Here, we report the crystal structure of MscTV at 1.6-A resolution. Unexpectedly, MscTV was found to be a water-soluble protein exhibiting a winged helix-turn-helix (wHTH) motif, which is the signature of the MarR (multiple antibiotic resistance regulator) family of transcriptional regulators. A cell-based osmotic downshock functional assay demonstrated that MscTV was unable to protect a knockout strain of Escherichia coli from hypoosmotic shock, further indicating that it does not function as a MS channel. We propose this protein be renamed MLPTv for MarR-like protein from T. volcanium.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125, USA.