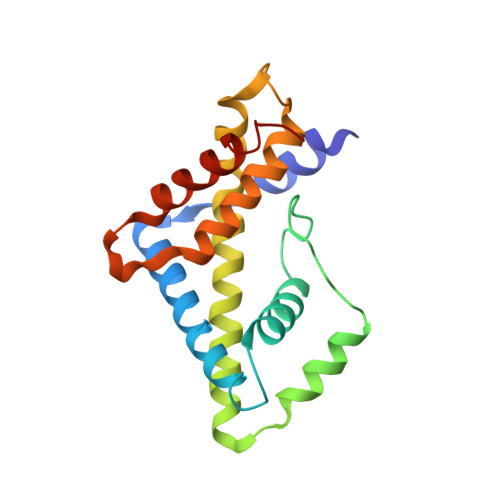
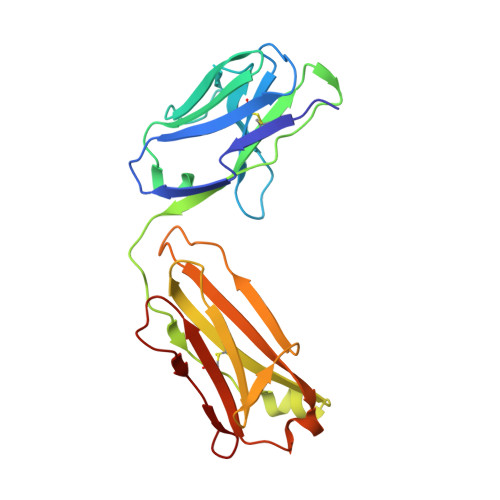
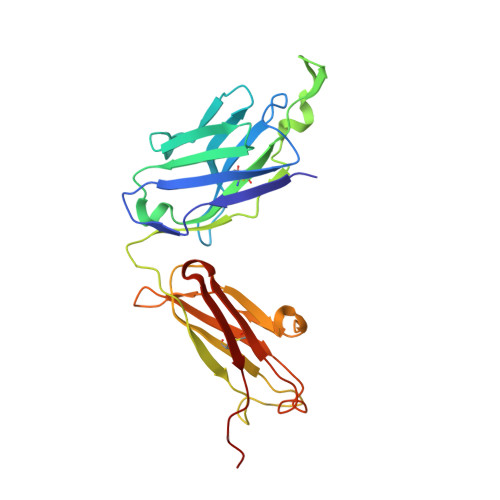
Elicitation of structure-specific antibodies by epitope scaffolds.
Ofek, G., Guenaga, F.J., Schief, W.R., Skinner, J., Baker, D., Wyatt, R., Kwong, P.D.(2010) Proc Natl Acad Sci U S A 107: 17880-17887
- PubMed: 20876137
- DOI: https://doi.org/10.1073/pnas.1004728107
- Primary Citation of Related Structures:
3LES, 3LEV, 3LEX, 3LEY - PubMed Abstract:
Elicitation of antibodies against targets that are immunorecessive, cryptic, or transient in their native context has been a challenge for vaccine design. Here we demonstrate the elicitation of structure-specific antibodies against the HIV-1 gp41 epitope of the broadly neutralizing antibody 2F5. This conformationally flexible region of gp41 assumes mostly helical conformations but adopts a kinked, extended structure when bound by antibody 2F5. Computational techniques were employed to transplant the 2F5 epitope into select acceptor scaffolds. The resultant "2F5-epitope scaffolds" possessed nanomolar affinity for antibody 2F5 and a range of epitope flexibilities and antigenic specificities. Crystallographic characterization of the epitope scaffold with highest affinity and antigenic discrimination confirmed good to near perfect attainment of the target conformation for the gp41 molecular graft in free and 2F5-bound states, respectively. Animals immunized with 2F5-epitope scaffolds showed levels of graft-specific immune responses that correlated with graft flexibility (p < 0.04), while antibody responses against the graft-as dissected residue-by-residue with alanine substitutions-resembled more closely those of 2F5 than sera elicited with flexible or cyclized peptides, a resemblance heightened by heterologous prime-boost. Lastly, crystal structures of a gp41 peptide in complex with monoclonal antibodies elicited by the 2F5-epitope scaffolds revealed that the elicited antibodies induce gp41 to assume its 2F5-recognized shape. Epitope scaffolds thus provide a means to elicit antibodies that recognize a predetermined target shape and sequence, even if that shape is transient in nature, and a means by which to dissect factors influencing such elicitation.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA.