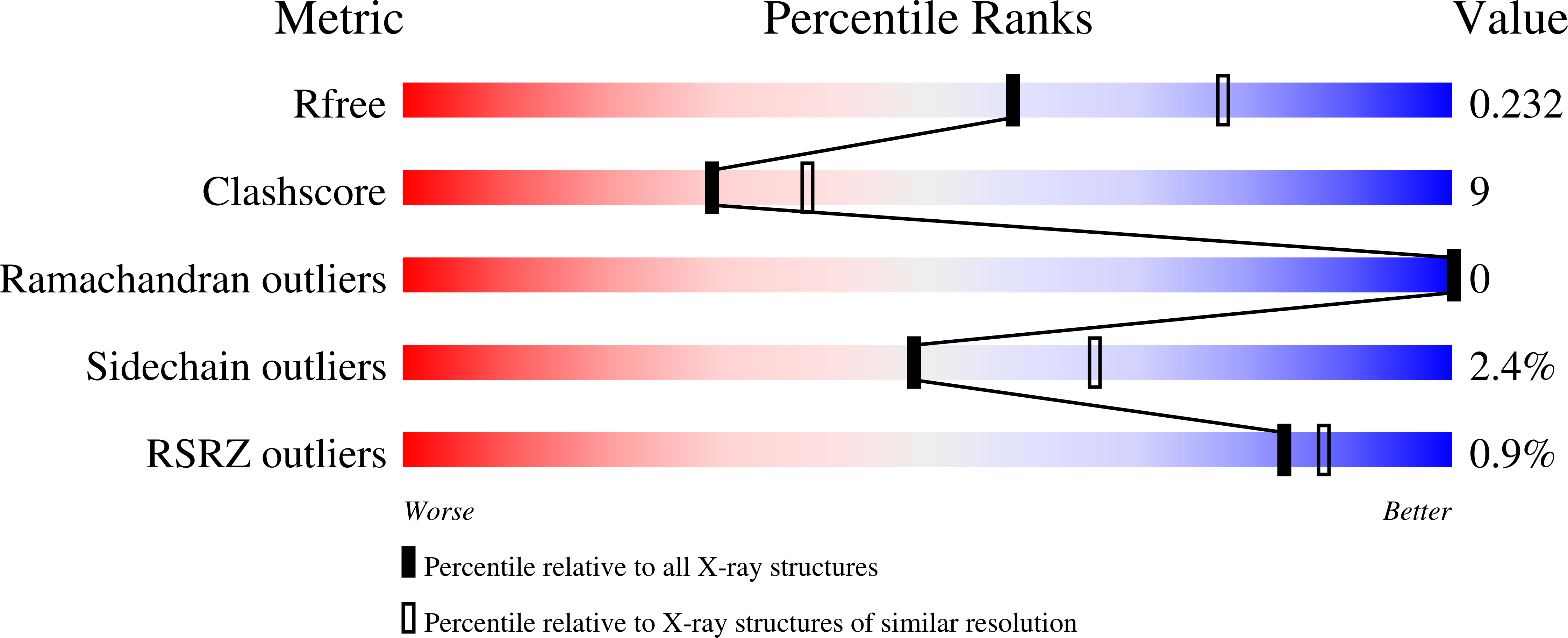
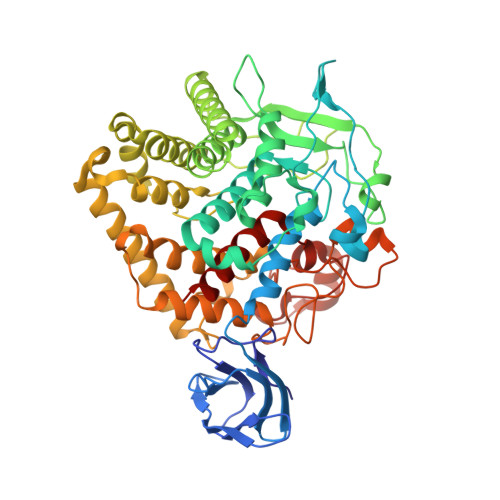
Structure of endoglucanase Cel9A from the thermoacidophilic Alicyclobacillus acidocaldarius
Pereira, J.H., Sapra, R., Volponi, J.V., Kozina, C.L., Simmons, B., Adams, P.D.(2009) Acta Crystallogr D Biol Crystallogr 65: 744-750
- PubMed: 19622857
- DOI: https://doi.org/10.1107/S0907444909012773
- Primary Citation of Related Structures:
3EZ8 - PubMed Abstract:
The production of biofuels using biomass is an alternative route to support the growing global demand for energy and to also reduce the environmental problems caused by the burning of fossil fuels. Cellulases are likely to play an important role in the degradation of biomass and the production of sugars for subsequent fermentation to fuel. Here, the crystal structure of an endoglucanase, Cel9A, from Alicyclobacillus acidocaldarius (Aa_Cel9A) is reported which displays a modular architecture composed of an N-terminal Ig-like domain connected to the catalytic domain. This paper describes the overall structure and the detailed contacts between the two modules. Analysis suggests that the interaction involving the residues Gln13 (from the Ig-like module) and Phe439 (from the catalytic module) is important in maintaining the correct conformation of the catalytic module required for protein activity. Moreover, the Aa_Cel9A structure shows three metal-binding sites that are associated with the thermostability and/or substrate affinity of the enzyme.
Organizational Affiliation:
Joint BioEnergy Institute, Emeryville, CA 94608, USA.