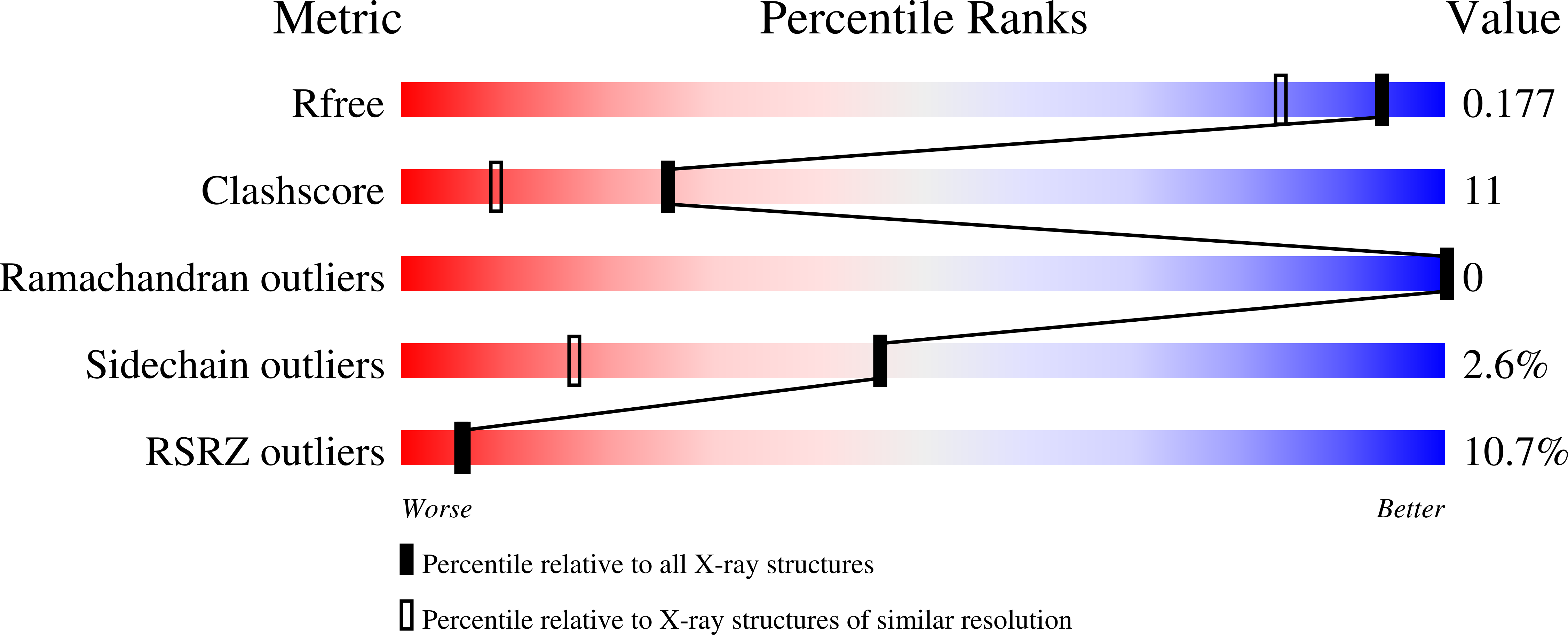
Large nucleotide-dependent conformational change in Rab28.
Lee, S.H., Baek, K., Dominguez, R.(2008) FEBS Lett 582: 4107-4111
- PubMed: 19026641
- DOI: https://doi.org/10.1016/j.febslet.2008.11.008
- Primary Citation of Related Structures:
3E5H - PubMed Abstract:
Rab GTPases are essential regulators of membrane trafficking. We report crystal structures of Rab28 in the active (GppNHp-bound) and inactive (GDP-3'P-bound) forms at 1.5 and 1.1A resolution. Rab28 is a distant member of the Rab family. While the overall fold of Rab28 resembles that of other Rab GTPases, it undergoes a larger nucleotide-dependent conformational change than other members of this family. Added flexibility resulting from a double-glycine motif at the beginning of switch 2 might partially account for this observation. The double-glycine motif, which is conserved in the Arf family, only occurs in Rab28 and Rab7B of the Rab family, and may have a profound effect on their catalytic activities.
Organizational Affiliation:
Department of Physiology, University of Pennsylvania School of Medicine, 3700 Hamilton Walk, A507 Richards Building, Philadelphia, PA 19104-6085, USA.