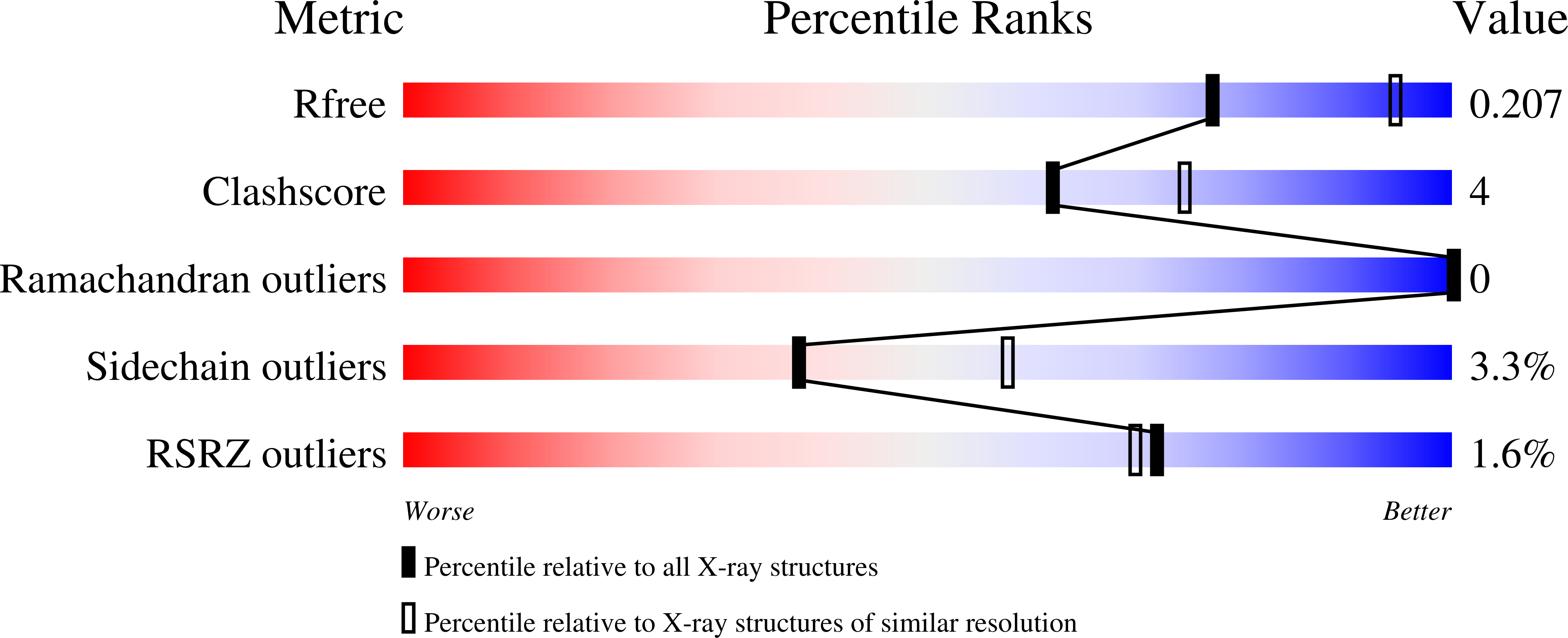
Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: crystal structure characterization with enzymatic inhibition assay
Zhang, L., Kong, Y., Wu, D., Zhang, H., Wu, J., Chen, J., Ding, J., Hu, L., Jiang, H., Shen, X.(2008) Protein Sci 17: 1971-1978
- PubMed: 18780820
- DOI: https://doi.org/10.1110/ps.036186.108
- Primary Citation of Related Structures:
3CF8, 3CF9, 3D04 - PubMed Abstract:
Flavonoids are the major functional components of many herbal and insect preparations and demonstrate varied pharmacological functions including antibacterial activity. Here by enzymatic assay and crystal structure analysis, we studied the inhibition of three flavonoids (quercetin, apigenin, and (S)-sakuranetin) against the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori (HpFabZ). These three flavonoids are all competitive inhibitors against HpFabZ by either binding to the entrance of substrate tunnel B (binding model A) or plugging into the tunnel C near the catalytic residues (binding model B) mainly by hydrophobic interaction and hydrogen-bond pattern. Surrounded by hydrophobic residues of HpFabZ at both positions of models A and B, the methoxy group at C-7 of (S)-sakuranetin seems to play an important role for the inhibitor's binding to HpFabZ, partly responsible for the higher inhibitory activity of (S)-sakuranetin than those of quercetin and apigenin against HpFabZ (IC(50) in microM: (S)-sakuranetin, 2.0 +/- 0.1; quercetin: 39.3 +/- 2.7; apigenin, 11.0 +/- 2.5). Our work is expected to supply useful information for understanding the potential antibacterial mechanism of flavonoids.
Organizational Affiliation:
Drug Discovery and Design Center, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.