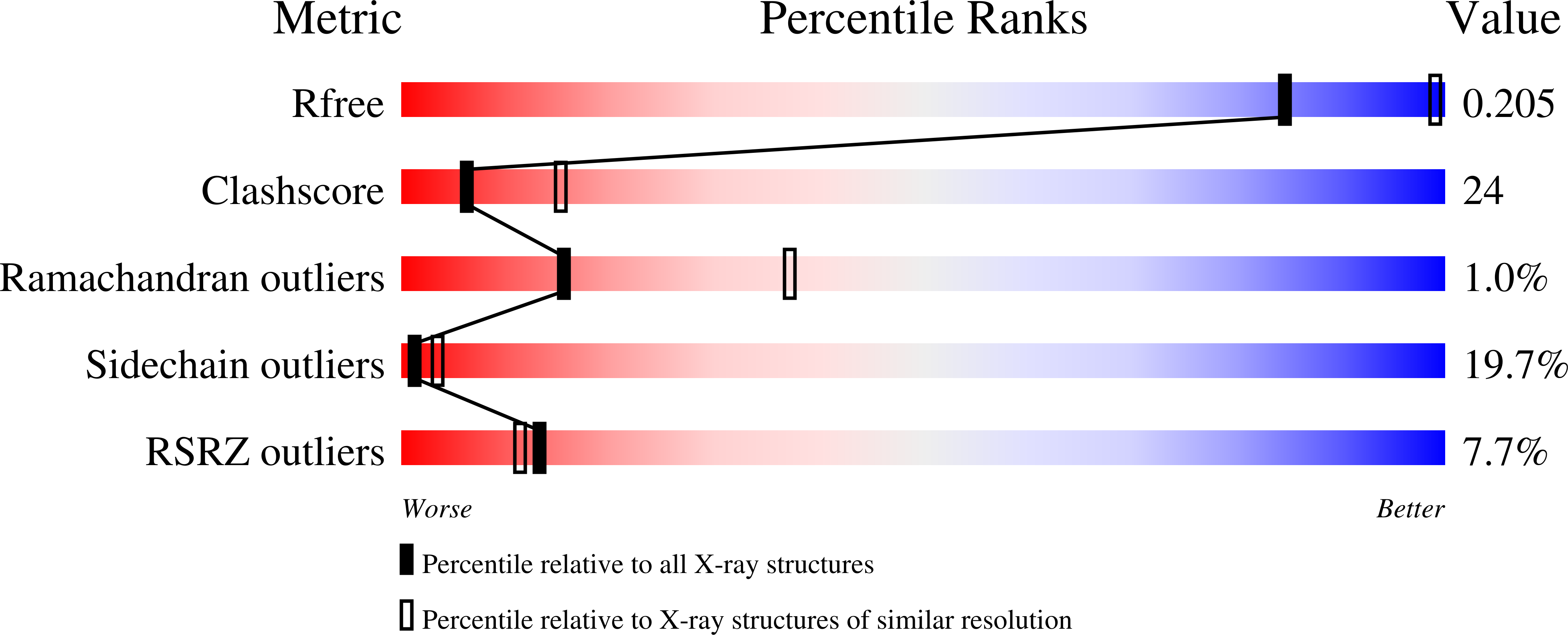
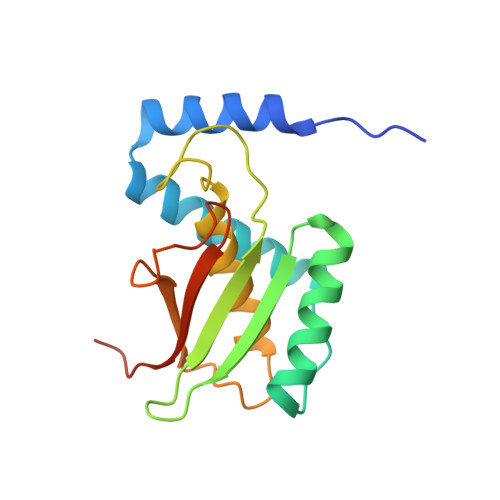
Structure of bacterial cellulose synthase subunit D octamer with four inner passageways
Hu, S.Q., Gao, Y.G., Tajima, K., Sunagawa, N., Zhou, Y., Kawano, S., Fujiwara, T., Yoda, T., Shimura, D., Satoh, Y., Munekata, M., Tanaka, I., Yao, M.(2010) Proc Natl Acad Sci U S A 107: 17957-17961
- PubMed: 20921370
- DOI: https://doi.org/10.1073/pnas.1000601107
- Primary Citation of Related Structures:
3A8E, 3AJ1, 3AJ2 - PubMed Abstract:
The cellulose synthesizing terminal complex consisting of subunits A, B, C, and D in Acetobacter xylinum spans the outer and inner cell membranes to synthesize and extrude glucan chains, which are assembled into subelementary fibrils and further into a ribbon. We determined the structures of subunit D (AxCeSD/AxBcsD) with both N- and C-terminal His(6) tags, and in complex with cellopentaose. The structure of AxCeSD shows an exquisite cylinder shape (height: ∼65 Å, outer diameter: ∼90 Å, and inner diameter: ∼25 Å) with a right-hand twisted dimer interface on the cylinder wall, formed by octamer as a functional unit. All N termini of the octamer are positioned inside the AxCeSD cylinder and create four passageways. The location of cellopentaoses in the complex structure suggests that four glucan chains are extruded individually through their own passageway along the dimer interface in a twisted manner. The complex structure also shows that the N-terminal loop, especially residue Lys6, seems to be important for cellulose production, as confirmed by in vivo assay using mutant cells with axcesD gene disruption and N-terminus truncation. Taking all results together, a model of the bacterial terminal complex is discussed.
Organizational Affiliation:
Faculty of Advanced Life Science, Hokkaido University, Sapporo 060-0810, Japan.