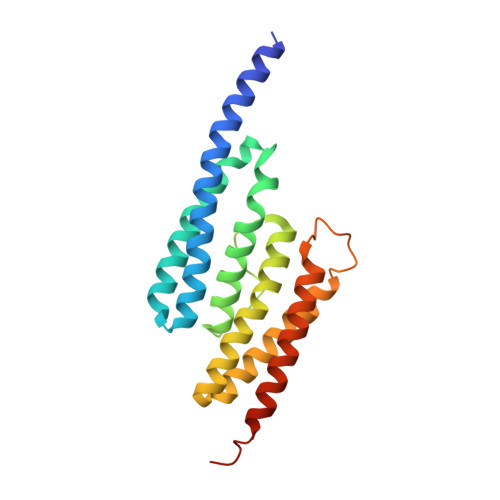
Structural Basis for Duplex RNA Recognition and Cleavage by Archaeoglobus Fulgidus C3Po.
Parizotto, E.A., Lowe, E.D., Parker, J.S.(2013) Nat Struct Mol Biol 20: 380
- PubMed: 23353787
- DOI: https://doi.org/10.1038/nsmb.2487
- Primary Citation of Related Structures:
3ZC0, 3ZC1 - PubMed Abstract:
Oligomeric complexes of Trax and Translin proteins, known as C3POs, participate in several eukaryotic nucleic acid metabolism pathways, including RNA interference and tRNA processing. In RNA interference in humans and Drosophila, C3PO activates the RNA-induced silencing complex (RISC) by removing the passenger strand of the small interfering RNA precursor duplex, using nuclease activity present in Trax. How C3POs engage with nucleic acid substrates is unknown. Here we identify a single protein from Archaeoglobus fulgidus that assembles into an octamer highly similar to human C3PO. The structure in complex with duplex RNA reveals that the octamer entirely encapsulates a single 13-base-pair RNA duplex inside a large inner cavity. Trax-like-subunit catalytic sites target opposite strands of the duplex for cleavage separated by 7 base pairs. The structure provides insight into the mechanism of RNA recognition and cleavage by an archaeal C3PO-like complex.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, Oxford, UK.