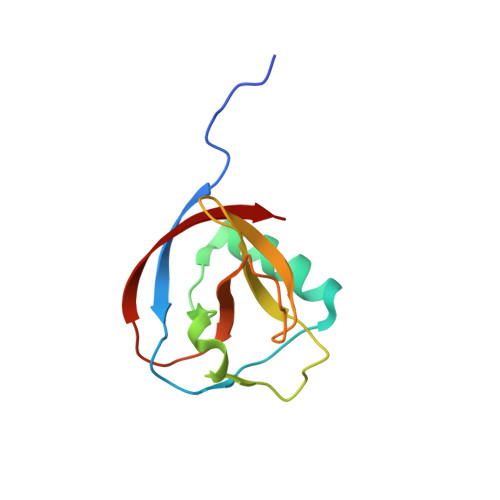
Structure of Alphacoronavirus Tgev Nsp1 Has Implications for Coronavirus Nsp1 Function and Evolution.
Jansson, A.M.(2013) J Virol 87: 2949
- PubMed: 23269811
- DOI: https://doi.org/10.1128/JVI.03163-12
- Primary Citation of Related Structures:
3ZBD - PubMed Abstract:
Coronavirus nsp1 has been shown to induce suppression of host gene expression and to interfere with the host immune response. However, the mechanism is currently unknown. The only available structural information on coronavirus nsp1 is the nuclear magnetic resonance (NMR) structure of the N-terminal domain of nsp1 from severe acute respiratory syndrome coronavirus (SARS-CoV) from the betacoronavirus genus. Here we present the first nsp1 structure from an alphacoronavirus, transmissible gastroenteritis virus (TGEV) nsp1. It displays a six-stranded β-barrel fold with a long alpha helix on the rim of the barrel, a fold shared with SARS-CoV nsp1(13-128). Contrary to previous speculation, the TGEV nsp1 structure suggests that coronavirus nsp1s have a common origin, despite the lack of sequence homology. However, comparisons of surface electrostatics, shape, and amino acid conservation between the alpha- and betacoronaviruses lead us to speculate that the mechanism for nsp1-induced suppression of host gene expression might be different in these two genera.
Organizational Affiliation:
Department of Cell and Molecular Biology, Uppsala University, Biomedical Center, Uppsala, Sweden. anna.jansson@icm.uu.se