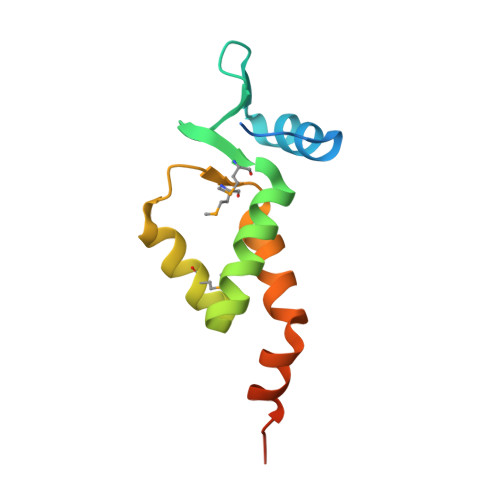
X-ray crystal structure of the N-terminal region of Moloney murine leukemia virus integrase and its implications for viral DNA recognition.
Guan, R., Aiyer, S., Cote, M.L., Xiao, R., Jiang, M., Acton, T.B., Roth, M.J., Montelione, G.T.(2017) Proteins 85: 647-656
- PubMed: 28066922
- DOI: https://doi.org/10.1002/prot.25245
- Primary Citation of Related Structures:
3NNQ, 4NZG - PubMed Abstract:
The retroviral integrase (IN) carries out the integration of a dsDNA copy of the viral genome into the host DNA, an essential step for viral replication. All IN proteins have three general domains, the N-terminal domain (NTD), the catalytic core domain, and the C-terminal domain. The NTD includes an HHCC zinc finger-like motif, which is conserved in all retroviral IN proteins. Two crystal structures of Moloney murine leukemia virus (M-MuLV) IN N-terminal region (NTR) constructs that both include an N-terminal extension domain (NED, residues 1-44) and an HHCC zinc-finger NTD (residues 45-105), in two crystal forms are reported. The structures of IN NTR constructs encoding residues 1-105 (NTR 1-105 ) and 8-105 (NTR 8-105 ) were determined at 2.7 and 2.15 Å resolution, respectively and belong to different space groups. While both crystal forms have similar protomer structures, NTR 1-105 packs as a dimer and NTR 8-105 packs as a tetramer in the asymmetric unit. The structure of the NED consists of three anti-parallel β-strands and an α-helix, similar to the NED of prototype foamy virus (PFV) IN. These three β-strands form an extended β-sheet with another β-strand in the HHCC Zn 2+ binding domain, which is a unique structural feature for the M-MuLV IN. The HHCC Zn 2+ binding domain structure is similar to that in HIV and PFV INs, with variations within the loop regions. Differences between the PFV and MLV IN NEDs localize at regions identified to interact with the PFV LTR and are compared with established biochemical and virological data for M-MuLV. Proteins 2017; 85:647-656. © 2016 Wiley Periodicals, Inc.
Organizational Affiliation:
Center for Advanced Biotechnology and Medicine, Rutgers, The State University of New Jersey, Piscataway, New Jersey, 08854.