Kinetic and structural characterization of DmpI from Helicobacter pylori and Archaeoglobus fulgidus, two 4-oxalocrotonate tautomerase family members.
Almrud, J.J., Dasgupta, R., Czerwinski, R.M., Kern, A.D., Hackert, M.L., Whitman, C.P.(2010) Bioorg Chem 38: 252-259
- PubMed: 20709352
- DOI: https://doi.org/10.1016/j.bioorg.2010.07.002
- Primary Citation of Related Structures:
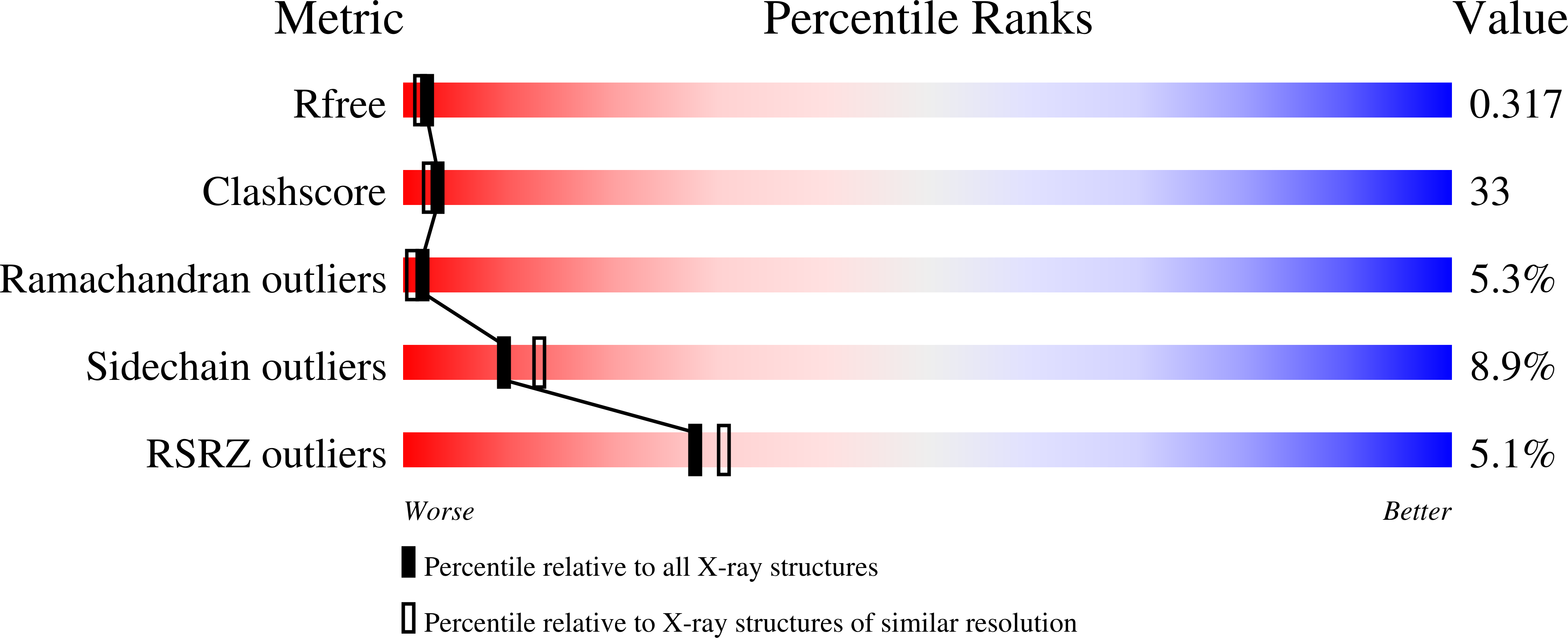
2ORM, 3M20, 3M21 - PubMed Abstract:
The tautomerase superfamily consists of structurally homologous proteins that are characterized by a β-α-β fold and a catalytic amino-terminal proline. 4-Oxalocrotonate tautomerase (4-OT) family members have been identified and categorized into five subfamilies on the basis of multiple sequence alignments and the conservation of key catalytic and structural residues. Representative members from two subfamilies have been cloned, expressed, purified, and subjected to kinetic and structural characterization. The crystal structure of DmpI from Helicobacter pylori (HpDmpI), a 4-OT homolog in subfamily 3, has been determined to high resolution (1.8Å and 2.1Å) in two different space groups. HpDmpI is a homohexamer with an active site cavity that includes Pro-1, but lacks the equivalent of Arg-11 and Arg-39 found in 4-OT. Instead, the side chain of Lys-36 replaces that of Arg-11 in a manner similar to that observed in the trimeric macrophage migration inhibitory factor (MIF), which is the title protein of another family in the superfamily. The electrostatic surface of the active site is also quite different and suggests that HpDmpI might prefer small, monoacid substrates. A kinetic analysis of the enzyme is consistent with the structural analysis, but a biological role for the enzyme remains elusive. The crystal structure of DmpI from Archaeoglobus fulgidus (AfDmpI), a 4-OT homolog in subfamily-4, has been determined to 2.4Å resolution. AfDmpI is also a homohexamer, with a proposed active site cavity that includes Pro-1, but lacks any other residues that are readily identified as catalytic ones related to 4-OT activity. Indeed, the electrostatic potential of the active site differs significantly in that it is mostly neutral, in contrast to the usual electropositive features found in other 4-OT family members, suggesting that AfDmpI might accommodate hydrophobic substrates. A kinetic analysis has been carried out, but does not provide any clues about the type of reaction the enzyme might catalyze.
Organizational Affiliation:
The University of Texas, Austin, 78712-1071, United States.