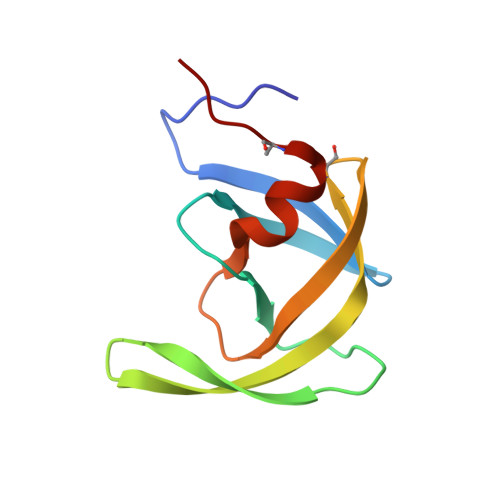
Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease.
Wlodawer, A., Miller, M., Jaskolski, M., Sathyanarayana, B.K., Baldwin, E., Weber, I.T., Selk, L.M., Clawson, L., Schneider, J., Kent, S.B.(1989) Science 245: 616-621
- PubMed: 2548279
- DOI: https://doi.org/10.1126/science.2548279
- Primary Citation of Related Structures:
3HVP - PubMed Abstract:
The rational design of drugs that can inhibit the action of viral proteases depends on obtaining accurate structures of these enzymes. The crystal structure of chemically synthesized HIV-1 protease has been determined at 2.8 angstrom resolution (R factor of 0.184) with the use of a model based on the Rous sarcoma virus protease structure. In this enzymatically active protein, the cysteines were replaced by alpha-amino-n-butyric acid, a nongenetically coded amino acid. This structure, in which all 99 amino acids were located, differs in several important details from that reported previously by others. The interface between the identical subunits forming the active protease dimer is composed of four well-ordered beta strands from both the amino and carboxyl termini and residues 86 to 94 have a helical conformation. The observed arrangement of the dimer interface suggests possible designs for dimerization inhibitors.
Organizational Affiliation:
Crystallography Laboratory, NCI-Frederick Cancer Research Facility, MD 21701.