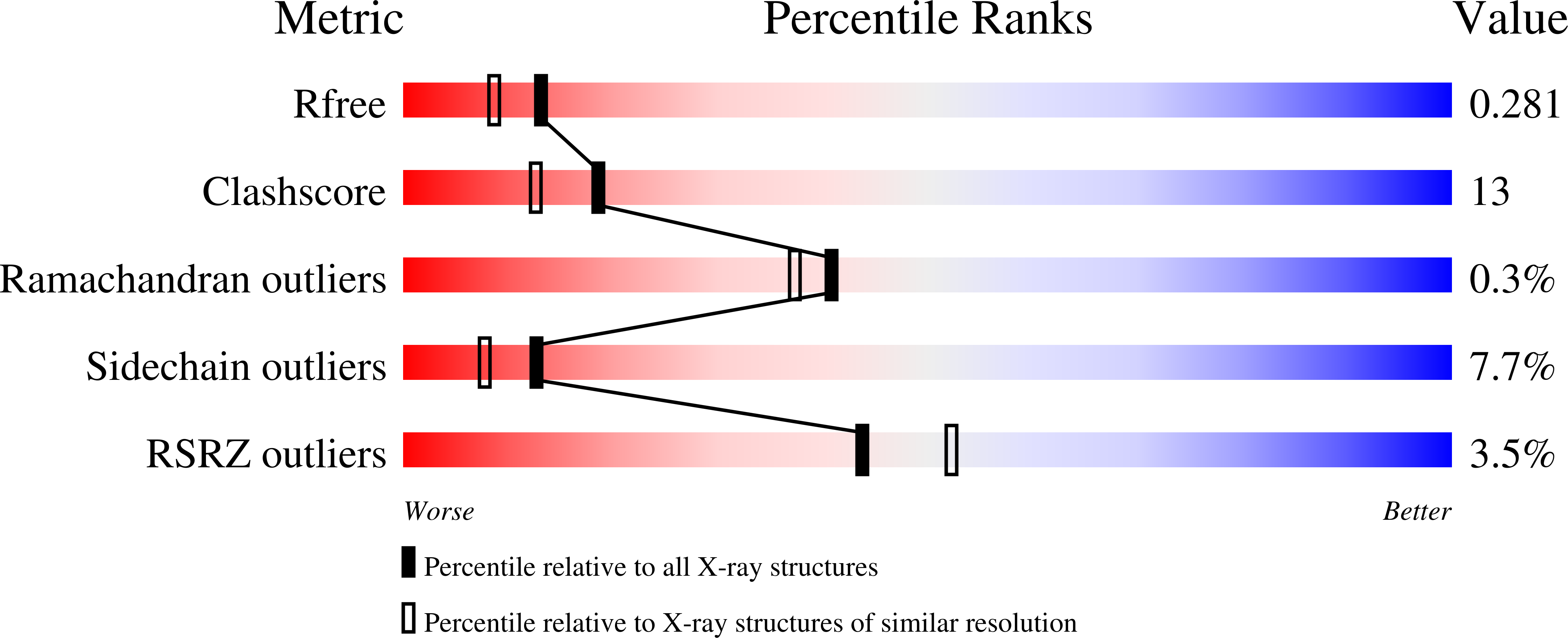
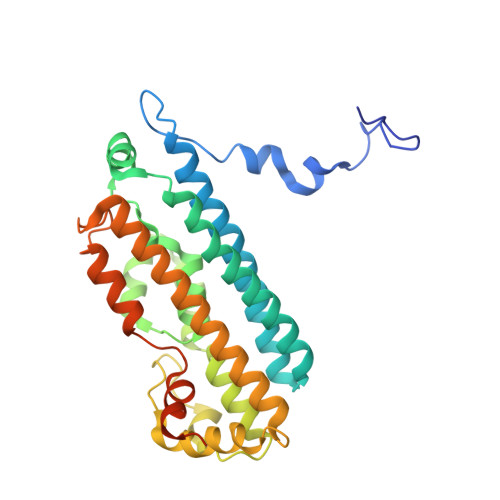
Histidine 55 of tryptophan 2,3-dioxygenase is not an active site base but regulates catalysis by controlling substrate binding
Thackray, S.J., Bruckmann, C., Anderson, J.L.R., Campbell, L.P., Xiao, R., Zhao, L., Mowat, C.G., Forouhar, F., Tong, L., Chapman, S.K.(2008) Biochemistry 47: 10677-10684
- PubMed: 18783250
- DOI: https://doi.org/10.1021/bi801202a
- Primary Citation of Related Structures:
3BK9, 3E08 - PubMed Abstract:
Tryptophan 2,3-dioxygenase (TDO) from Xanthomonas campestris is a highly specific heme-containing enzyme from a small family of homologous enzymes, which includes indoleamine 2,3-dioxygenase (IDO). The structure of wild type (WT TDO) in the catalytically active, ferrous (Fe (2+)) form and in complex with its substrate l-tryptophan ( l-Trp) was recently reported [Forouhar et al. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 473-478] and revealed that histidine 55 hydrogen bonds to l-Trp, precisely positioning it in the active site and implicating it as a possible active site base. In this study the substitution of the active site residue histidine 55 by alanine and serine (H55A and H55S) provides insight into the molecular mechanism used by the enzyme to control substrate binding. We report the crystal structure of the H55A and H55S mutant forms at 2.15 and 1.90 A resolution, respectively, in binary complexes with l-Trp. These structural data, in conjunction with potentiometric and kinetic studies on both mutants, reveal that histidine 55 is not essential for turnover but greatly disfavors the mechanistically unproductive binding of l-Trp to the oxidized enzyme allowing control of catalysis. This is demonstrated by the difference in the K d values for l-Trp binding to the two oxidation states of wild-type TDO (3.8 mM oxidized, 4.1 microM reduced), H55A TDO (11.8 microM oxidized, 3.7 microM reduced), and H55S TDO (18.4 microM oxidized, 5.3 microM reduced).
Organizational Affiliation:
EaStCHEM, School of Chemistry, University of Edinburgh, UK.