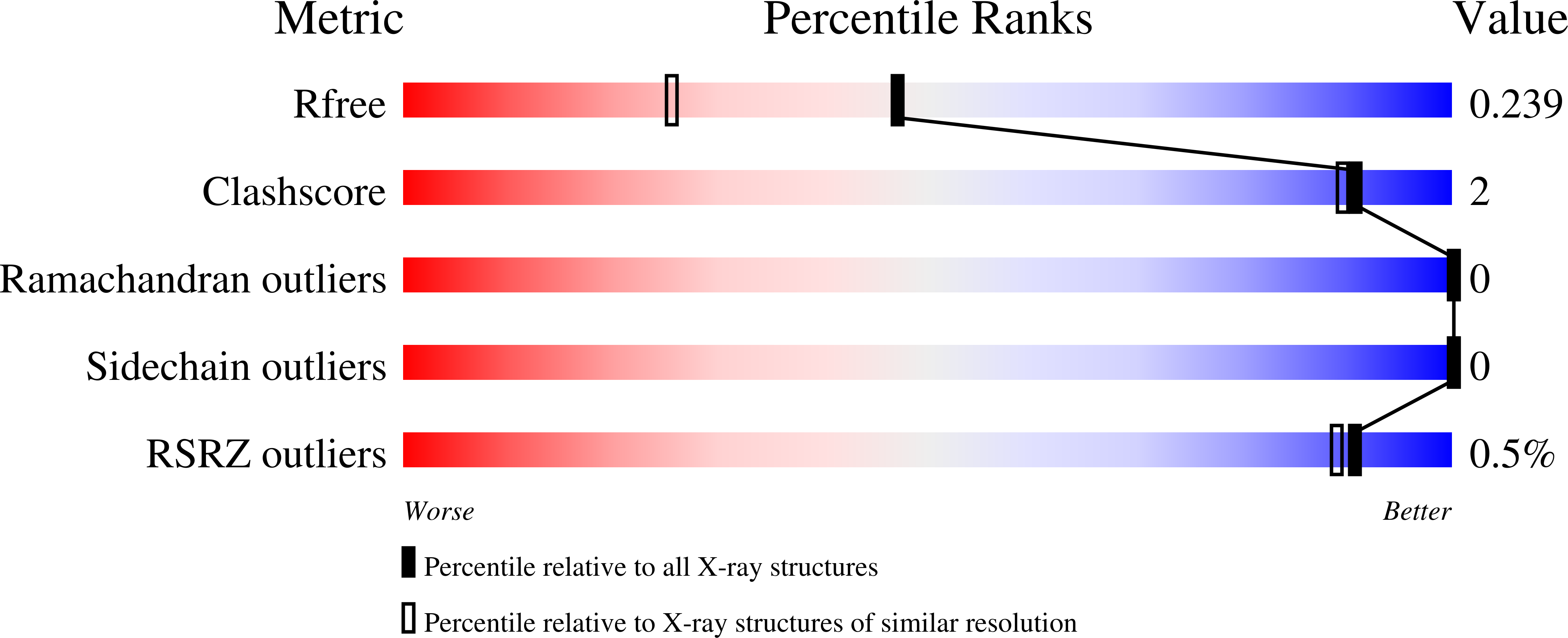
De novo computational design of retro-aldol enzymes.
Jiang, L., Althoff, E.A., Clemente, F.R., Doyle, L., Rothlisberger, D., Zanghellini, A., Gallaher, J.L., Betker, J.L., Tanaka, F., Barbas, C.F., Hilvert, D., Houk, K.N., Stoddard, B.L., Baker, D.(2008) Science 319: 1387-1391
- PubMed: 18323453
- DOI: https://doi.org/10.1126/science.1152692
- Primary Citation of Related Structures:
3B5L, 3HOJ - PubMed Abstract:
The creation of enzymes capable of catalyzing any desired chemical reaction is a grand challenge for computational protein design. Using new algorithms that rely on hashing techniques to construct active sites for multistep reactions, we designed retro-aldolases that use four different catalytic motifs to catalyze the breaking of a carbon-carbon bond in a nonnatural substrate. Of the 72 designs that were experimentally characterized, 32, spanning a range of protein folds, had detectable retro-aldolase activity. Designs that used an explicit water molecule to mediate proton shuffling were significantly more successful, with rate accelerations of up to four orders of magnitude and multiple turnovers, than those involving charged side-chain networks. The atomic accuracy of the design process was confirmed by the x-ray crystal structure of active designs embedded in two protein scaffolds, both of which were nearly superimposable on the design model.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195, USA.