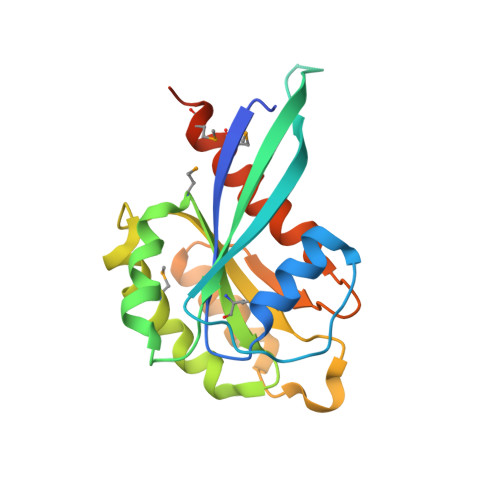
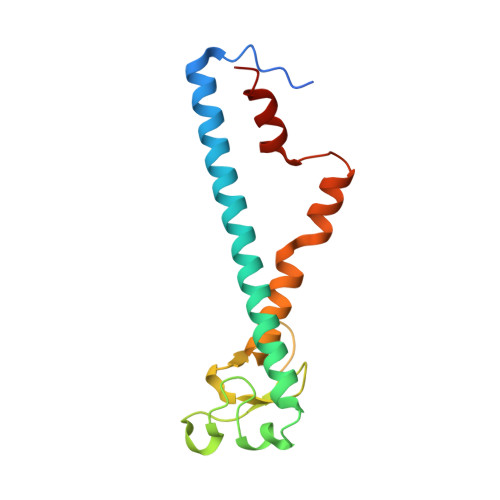
Structural basis for the exclusive specificity of Slac2-a/melanophilin for the Rab27 GTPases.
Kukimoto-Niino, M., Sakamoto, A., Kanno, E., Hanawa-Suetsugu, K., Terada, T., Shirouzu, M., Fukuda, M., Yokoyama, S.(2008) Structure 16: 1478-1490
- PubMed: 18940604
- DOI: https://doi.org/10.1016/j.str.2008.07.014
- Primary Citation of Related Structures:
2ZET - PubMed Abstract:
Rab27A is required for actin-based melanosome transport in mammalian skin melanocytes through its interaction with a specific effector, Slac2-a/melanophilin. Mutations that disrupt the Rab27A/Slac2-a interaction cause human Griscelli syndrome. The other Rab27 isoform, Rab27B, also binds all of the known effectors of Rab27A. In this study, we determined the crystal structure of the constitutively active form of Rab27B complexed with GTP and the effector domain of Slac2-a. The Rab27B/Slac2-a complex exhibits several intermolecular hydrogen bonds that were not observed in the previously reported Rab3A/rabphilin complex. A Rab27A mutation that disrupts one of the specific hydrogen bonds with Slac2-a resulted in the dramatic reduction of Slac2-a binding activity. Furthermore, we generated a Rab3A mutant that acquires Slac2-a binding ability by transplanting four Rab27-specific residues into Rab3A. These findings provide the structural basis for the exclusive association of Slac2-a with the Rab27 subfamily, whereas rabphilin binds several subfamilies, including Rab3 and Rab27.
Organizational Affiliation:
Systems and Structural Biology Center, Yokohama Institute, RIKEN, 1-7-22 Suehiro-cho, Tsurumi, Yokohama 230-0045, Japan.