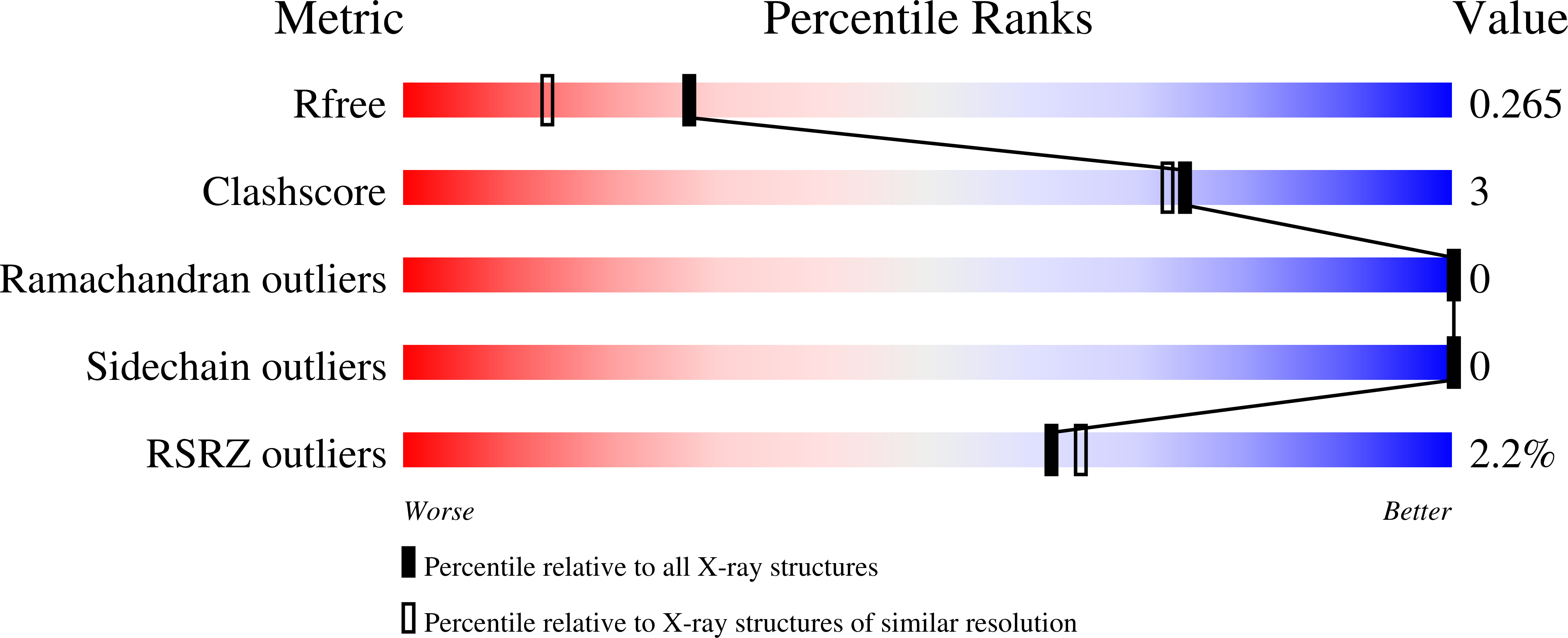
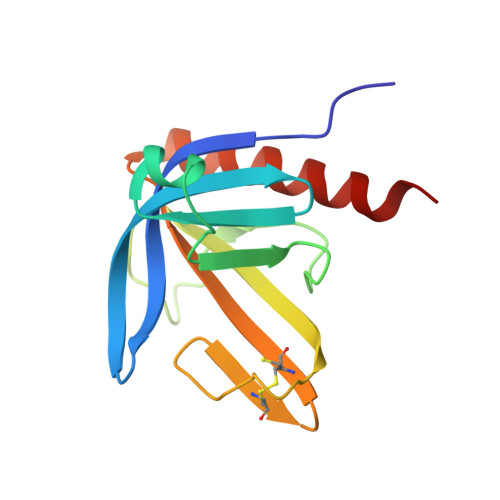
Crystal Structure of the Ph Domain of Human Actin-Binding Protein Anillin Anln
Vollmar, M., Wang, J., Krojer, T., Elkins, J., Filippakopoulos, P., Ugochukwu, E., Cocking, R., von Delft, F., Bountra, C., Arrowsmith, C.H., Weigelt, J., Edwards, A., Knapp, S.To be published.