The structural basis for catalysis and substrate specificity of a rhomboid protease.
Vinothkumar, K.R., Strisovsky, K., Andreeva, A., Christova, Y., Verhelst, S., Freeman, M.(2010) EMBO J 29: 3797-3809
- PubMed: 20890268
- DOI: https://doi.org/10.1038/emboj.2010.243
- Primary Citation of Related Structures:
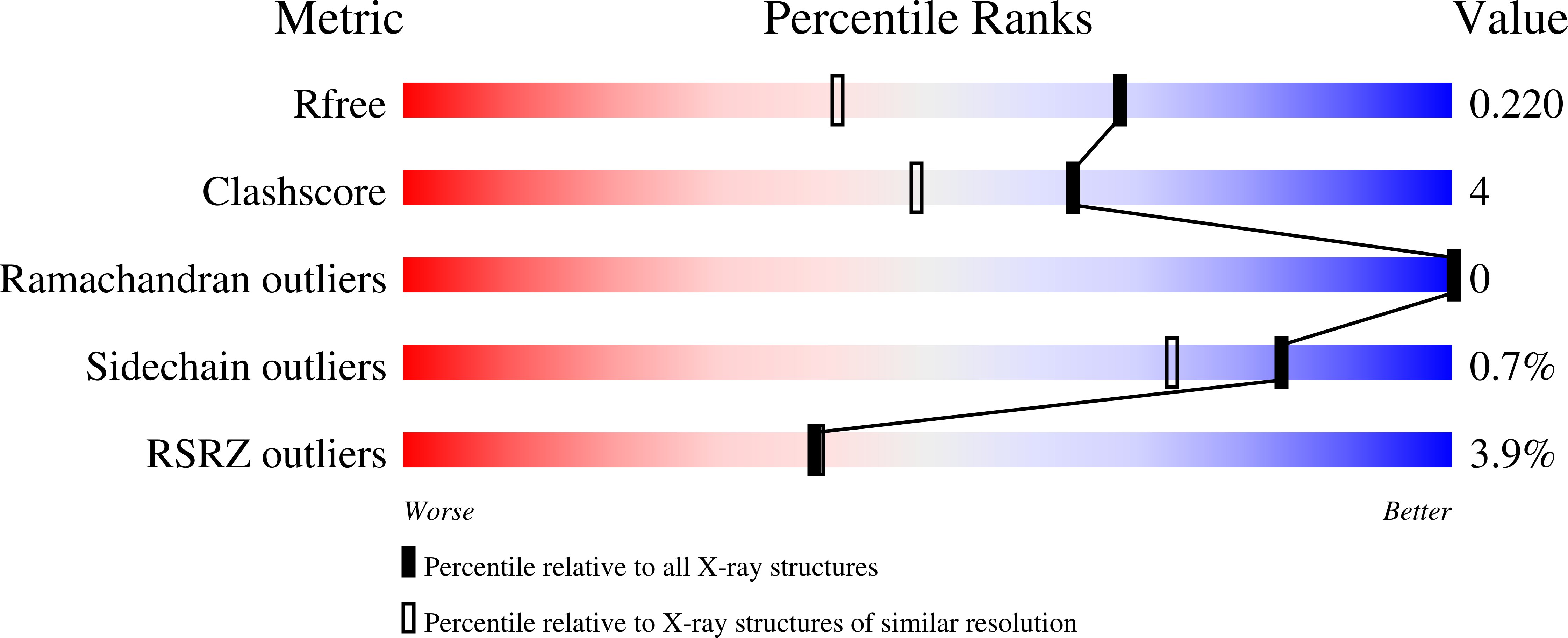
2XOV, 2XOW - PubMed Abstract:
Rhomboids are intramembrane proteases that use a catalytic dyad of serine and histidine for proteolysis. They are conserved in both prokaryotes and eukaryotes and regulate cellular processes as diverse as intercellular signalling, parasitic invasion of host cells, and mitochondrial morphology. Their widespread biological significance and consequent medical potential provides a strong incentive to understand the mechanism of these unusual enzymes for identification of specific inhibitors. In this study, we describe the structure of Escherichia coli rhomboid GlpG covalently bound to a mechanism-based isocoumarin inhibitor. We identify the position of the oxyanion hole, and the S₁- and S₂'-binding subsites of GlpG, which are the key determinants of substrate specificity. The inhibitor-bound structure suggests that subtle structural change is sufficient for catalysis, as opposed to large changes proposed from previous structures of unliganded GlpG. Using bound inhibitor as a template, we present a model for substrate binding at the active site and biochemically test its validity. This study provides a foundation for a structural explanation of rhomboid specificity and mechanism, and for inhibitor design.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge, UK. vkumar@mrc-lmb.cam.ac.uk