Structures of Two Truncated Phage-Tail Hyaluronate Lyases from Streptococcus Pyogenes Serotype M1.
Martinez-Fleites, C., Smith, N.L., Turkenburg, J.P., Black, G.W., Taylor, E.J.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 963
- PubMed: 19850999
- DOI: https://doi.org/10.1107/S1744309109032813
- Primary Citation of Related Structures:
2WB3, 2WH7 - PubMed Abstract:
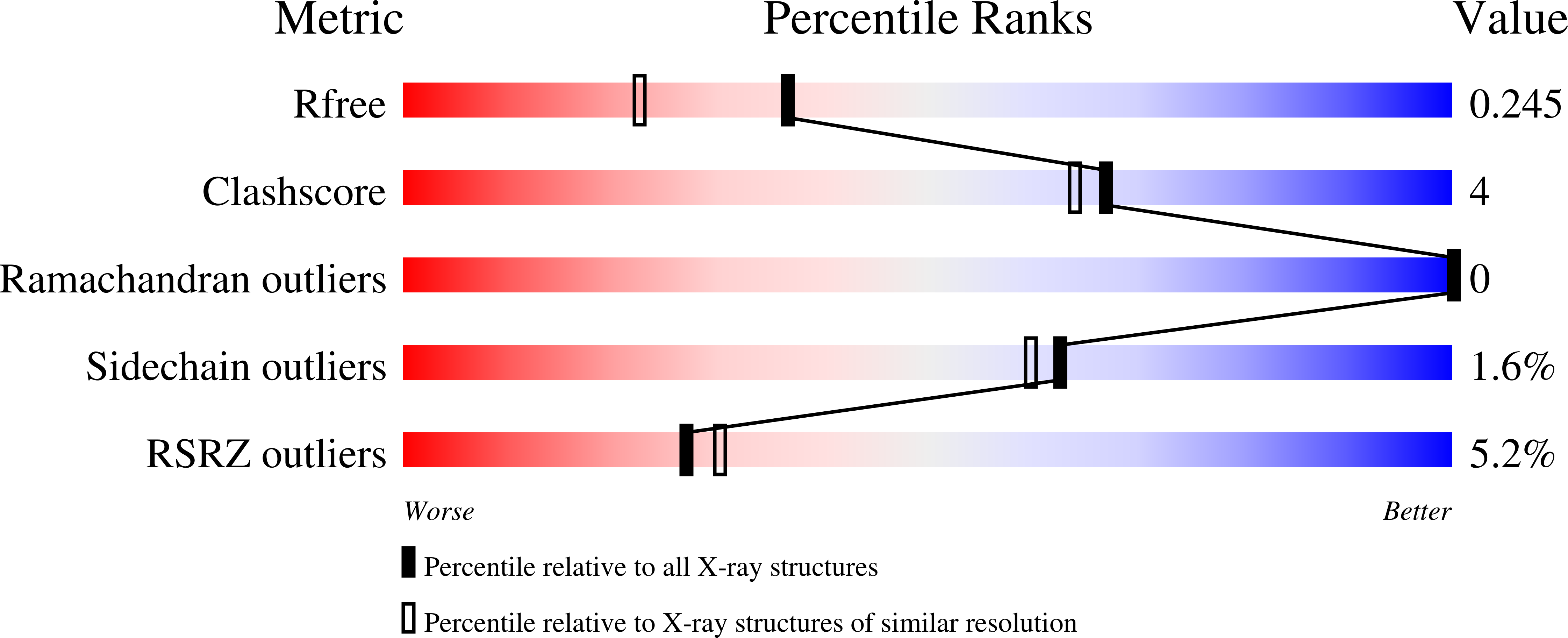
The crystal structures of truncated forms of the Streptococcus pyogenes phage-encoded hyaluronate lyases HylP2 and HylP3 were determined by molecular replacement to 1.6 and 1.9 A resolution, respectively. The truncated forms crystallized in a hexagonal space group, forming a trimer around the threefold crystallographic axis. The arrangement of the fold is very similar to that observed in the structure of the related hyaluronate lyase HylP1. The structural elements putatively involved in substrate recognition are found to be conserved in both the HylP2 and HylP3 fragments.
Organizational Affiliation:
Structural Biology Laboratory, Department of Chemistry, The University of York, York YO10 5YW, England.