High-Affinity Ige Recognition of a Conformational Epitope of the Major Respiratory Allergen Phl P 2 as Revealed by X-Ray Crystallography.
Padavattan, S., Flicker, S., Schirmer, T., Madritsch, C., Randow, S., Reese, G., Vieths, S., Lupinek, C., Ebner, C., Valenta, R., Markovic-Housley, Z.(2009) J Immunol 182: 2141
- PubMed: 19201867
- DOI: https://doi.org/10.4049/jimmunol.0803018
- Primary Citation of Related Structures:
2VXQ - PubMed Abstract:
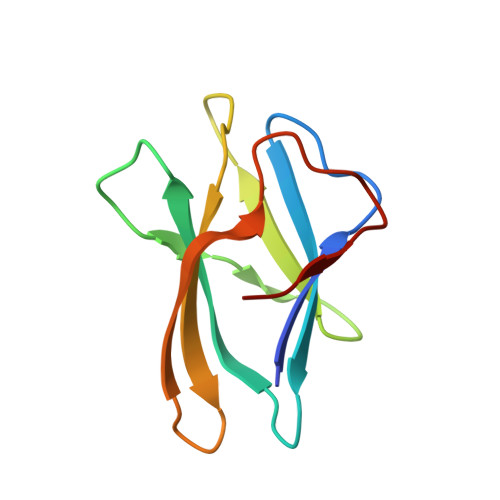
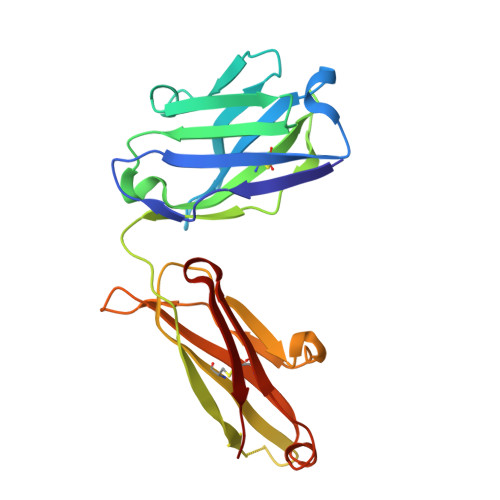
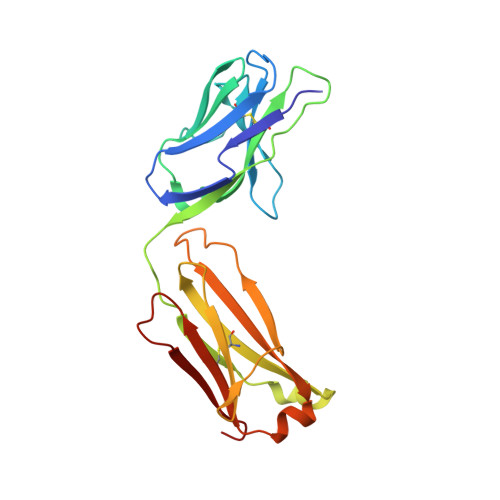
We report the three-dimensional structure of the complex between the major respiratory grass pollen allergen Phl p 2 and its specific human IgE-derived Fab. The Phl p 2-specific human IgE Fab has been isolated from a combinatorial library constructed from lymphocytes of a pollen allergic patient. When the variable domains of the IgE Fab were grafted onto human IgG1, the resulting Ab (huMab2) inhibited strongly the binding of allergic patients' IgE to Phl p 2 as well as allergen-induced basophil degranulation. Analysis of the binding of the allergen to the Ab by surface plasmon resonance yielded a very low dissociation constant (K(D) = 1.1 x 10(-10) M), which is similar to that between IgE and Fcepsilon;RI. The structure of the Phl p 2/IgE Fab complex was determined by x-ray crystallography to 1.9 A resolution revealing a conformational epitope (876 A(2)) comprised of the planar surface of the four-stranded anti-parallel beta-sheet of Phl p 2. The IgE-defined dominant epitope is discontinuous and formed by 21 residues located mostly within the beta strands. Of the 21 residues, 9 interact directly with 5 of the 6 CDRs (L1, L3, H1, H2, H3) of the IgE Fab predominantly by hydrogen bonding and van der Waals interactions. Our results indicate that IgE Abs recognize conformational epitopes with high affinity and provide a structural basis for the highly efficient effector cell activation by allergen/IgE immune complexes.
Organizational Affiliation:
Division of Structural Biology, Biozentrum, University of Basel, Basel, Switzerland.